The global pharmaceutical industry continues to see rising demand for high-quality Active Pharmaceutical Ingredients (APIs) that meet international regulatory standards. Among these, Travoprost, a prostaglandin F2α analogue used in treating glaucoma and ocular hypertension, has gained prominence in ophthalmic formulations. For companies seeking to expand or launch glaucoma-related products in regulated markets like the US, Europe, and the Middle East, the selection of a trustworthy Travoprost API supplier is mission-critical.
This is where Chemignition Laboratory stands out as a globally trusted, GMP-certified manufacturer and exporter of Travoprost API. In this in-depth article, we explore what makes Chemignition a preferred API partner and why it should be your go-to Travoprost API supplier.
1. Specialized Focus on Ophthalmic APIs
Chemignition has carved a niche in producing ophthalmic-grade APIs, including:
This specialized focus ensures in-depth understanding of the formulation sensitivity, regulatory requirements, and storage conditions essential for ophthalmic APIs. Unlike general-purpose API manufacturers, Chemignition brings precision, quality, and consistency that meets the standards of eye care products.
2. GMP-Certified Manufacturing Facility
At the core of Chemignition’s credibility lies its GMP-compliant manufacturing facility located in India’s leading industrial zone. The plant is equipped with:
- State-of-the-art synthesis and purification labs
- Automated process controls for consistency
- HEPA-filtered cleanroom environments
- In-house analytical and stability testing labs
All operations follow Good Manufacturing Practices (GMP) as outlined by global health authorities, ensuring batch-to-batch consistency and traceability.
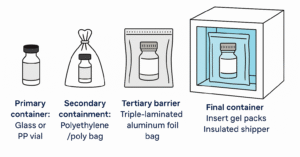
3. Expertise in Cold-Chain Logistics
Travoprost is a temperature-sensitive compound that requires storage between -20°C to -10°C to retain its potency. Chemignition Laboratory ensures safe transportation with:
- Cold-chain insulated packaging systems
- Real-time temperature data loggers
- Triple-layered, leak-proof containment
- Validated shipping protocols for international air and sea routes
With experience handling API exports to USA, Europe, Middle East, and Southeast Asia, Chemignition Laboratory guarantees cold-chain compliance from dispatch to delivery.
4. Global Export Readiness
Chemignition is an export-approved pharmaceutical manufacturer, having completed shipments and regulatory procedures across over 30+ countries. The team is well-versed in:
- Customs clearance and product coding
- Regulatory registrations in destination countries
- Export documentation (COO, Invoice, Packing List, Bill of Lading)
- Phytosanitary and shipping certificates
- Third-party inspection and pre-shipment validation
Chemignition Laboratory works with licensed freight forwarders to ensure that your Travoprost API arrives securely, on time, and with zero customs hurdles.
5. High Purity and Custom Specifications
The efficacy of ophthalmic drugs depends heavily on the purity and particle size distribution of the Travoprost API. Chemignition Laboratory offers:
- Purity >99% by HPLC
- Particle size control and micronization (if required)
- Customization of API form (e.g., anhydrous)
- Batch-wise impurity profiling
- Addition test as per customer requirements
Each API batch is tested in-house and by accredited third-party labs, ensuring confidence in every delivery.
6. Fast Response and Transparent Communication
One of the top pain points in pharmaceutical procurement is poor vendor communication. Chemignition prioritizes fast turnaround times with:
- 24–48 hour quotation timelines
- Transparent pricing with breakdowns
- Dedicated export managers for different countries
- Post-sale support for audits, samples, and clarifications
Buyers consistently appreciate the company’s clarity, professionalism, and speed in handling both commercial and technical queries.
7. Flexible Order Quantities & Contract Manufacturing
Whether you’re sourcing pilot batches for R&D or need bulk commercial-scale supply, Chemignition supports:
- Low MOQ flexibility for early-stage formulations
- Bulk orders with custom packaging options
- Contract manufacturing (CMO) partnerships for long-term projects
- Private-label packaging for white-label distribution partners
This makes it easier for both startups and large pharma companies to collaborate without operational friction.
8. Batch Documentation & Quality Assurance
Every Travoprost API batch from Chemignition Laboratory comes with a detailed dossier, which includes:
- CoA (Certificate of Analysis)
- MOA (Method of Analysis)
- MSDS (Material Safety Data Sheet)
- TSE/BSE Declaration
- Residual solvent and heavy metal testing reports
- Microbial limit tests (if applicable)
Chemignition’s internal QA/QC team also audits suppliers of raw materials to maintain end-to-end traceability and compliance.
9. R&D-Backed Innovation
Chemignition’s in-house R&D center is actively involved in:
- Optimizing yield and purity of Travoprost API
- Reducing impurity levels and synthesis cycle time
- Validating new packaging and storage formats
- Supporting customers with formulation and compatibility studies
This scientific support ensures formulators and pharma R&D teams receive continuous improvement on quality and stability.
10. Trusted by Global Buyers
Over the past years, Chemignition has built a loyal client base, including:
- Generic formulators in Europe and the US
- Contract manufacturers in the Middle East
- OTC product companies in Africa and Southeast Asia
- B2B API distributors in Latin America
Repeat orders and referrals demonstrate long-term trust and satisfaction in Chemignition’s supply chain management and product performance.
11. Why Travoprost API Buyers Prefer Chemignition Laboratory
| Feature | Chemignition Advantage |
|---|---|
| Certification | GMP, ISO, Regulatory Documentation Ready |
| Regulatory | CoA, SDS, TSE/BSE, MOA |
| Export Readiness | 30+ countries, customs support, cold-chain shipping |
| Storage & Packaging | Validated cold-chain, triple-layered HDPE/Aluminum |
| MOQ & Scalability | R&D batches to commercial scale available |
| Communication | Fast, responsive, and expert-level support |
| Specialization | Ophthalmic APIs like Travoprost, Bimatoprost, Latanoprost |
Ready to Source Travoprost API from Chemignition?
If you’re looking for a GMP-compliant, regulatory-ready, export-capable Travoprost API supplier, Chemignition Laboratory is your ideal partner.
Whether you’re in the early development phase or planning a commercial launch, our team can support:
- Sample dispatch and COA submission
- DMF or ASMF referencing
- Custom labeling and cold-chain logistics
- Bulk supply with flexible timelines
Contact Us Today
🌐 Website: [www.chemignition.com]
Final Thoughts
In the highly competitive and regulated world of ophthalmic APIs, Chemignition Laboratory provides the rare combination of technical excellence, export expertise, and regulatory preparedness. For companies aiming to build a trusted glaucoma product line, partnering with a reliable Travoprost API supplier like Chemignition Laboratory ensures compliance, consistency, and commercial success.