Key Properties of Bimatoprost
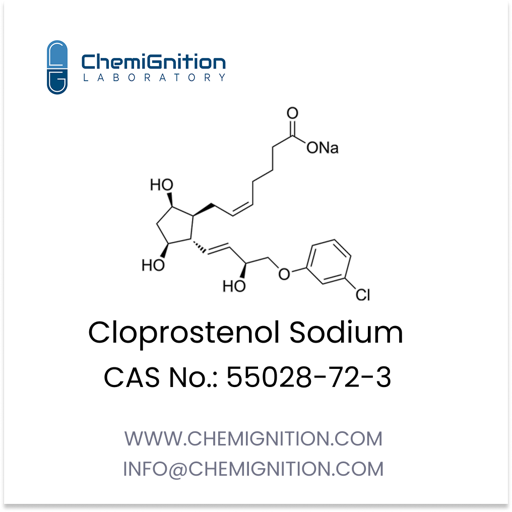
| Product Name: | Bimatoprost |
| Synonyms: | (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide |
| CAS No: | 155206-00-1 |
| Chemical Formula: | C₂₅H₃₇NO₄ |
| Grade: | USP/IP/IH |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 415.58 g/mol |
| Solubility: | Practically insoluble in water, but it is soluble in organic solvents such as ethanol and methanol |
Manufacturing process of Bimatoprost
The manufacturing process begins with ensuring equipment cleanliness and availability of utilities, raw materials, and packing materials.
Charge dichloromethane or toluene into a glass pot and add 17-phenyl trinor prostaglandin F2α methyl ester and an amine derivative are under stirring. The reaction temperature is maintained at 20–25°C during the addition, then adjusted to 30–35°C for 240–360 minutes to initiate and complete the reaction.
Take In-process sample from reaction mass and send the in-process sample to Quality control (QC) for reaction monitoring by HPLC. after results complies cool the reaction mass up to 20–25°C, an aqueous solution is added to quench the reaction, and the organic layer is separated.
The organic layer is concentrated under reduced pressure to obtain a crude product, which is purified through chromatography. The purified material is dissolved in a solvent, crystallized at low temperatures (0–5°C), filtered, and washed.
Pack Bimatoprost in a glass or polypropylene (PP) vial. Place the vial inside a poly bag, twist its mouth, and secure it with a PVC strip. Insert this bag into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Bimatoprost in a tightly closed, light-resistant container at a temperature of -20°C to -10°C.
Applications of Bimatoprost
Technical Specifications of Bimatoprost
| Sr.No. | Test | Specification |
|---|---|---|
| 01 | Description | White to off white powder |
| 02 | Solubility | Freely soluble in methanol and acetone |
| 03 | Identification | A) By IR: Infrared absorption spectrum obtained from the sample should correspond to the Bimatoprost reference standard spectrum. B) By HPLC: The retention time of the principal peak in the chromatogram of the sample preparation should match with that of standard preparation as obtained in assay test. |
| 04 | Water Content | Not more than 1.0% |
| 05 | Specific Optical Rotation | +33° – +38° |
| 06 | Related Substances by HPLC | A) P-toluene sulfonic acid: Not more than 0.15% B) Bimatoprost oxirane: Not more than 0.15% C) 15-epibimatoprost: Not more than 0.70% D) Bimatoprost related compound A: Not more than 0.50% E) T15-keto Bimatoprost: Not more than 0.20% F) Bimatoprost Acid: Not more than 0.10% G) Any individual impurity: Not more than 0.10% H) Total impurities: Not more than 1.20% |
| 07 | Assay by HPLC | Not less than 98.0% w/w to not more than 102.0% w/w |
| 08 | Residual Solvent by GC | A) Methanol: Not more than 3000 ppm B) Dichloromethane: Not more than 600 ppm C) Methyl tertiary-butyl ether: Not more than 5000 ppm D) Ethyl acetate: Not more than 5000 ppm E) Tetrahydrofuran: Not more than 720 ppm |
Packing and Storage condition of Bimatoprost
Pack Bimatoprost in a glass or polypropylene (PP) vial. Place the vial inside a poly bag, twist its mouth, and secure it with a PVC strip. Insert this bag into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Bimatoprost in a tightly closed, light-resistant container at a temperature of -20°C to -10°C.
Why Choose Chemignition for
Bimatoprost Supply?
Quality Assurance
Grade Compliance
Bimatoprost is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.
Large-Scale Manufacturing and Export
Grade Compliance
Bimatoprost is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.