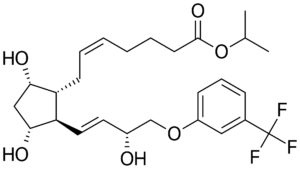
The demand for import Travoprost API, a key active ingredient in glaucoma and ocular hypertension medications, continues to rise in regulated markets like the United States and Europe. However, successfully importing this high-value ophthalmic API requires strict adherence to regulatory guidelines, documentation standards, and GMP protocols.
In this Travoprost API import guide, we’ll walk you through everything pharmaceutical companies, distributors, and CMOs need to know—from regulatory documentation (DMF, ASMF) to customs clearance and GMP certification.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
1. Why Compliance Matters in Travoprost API Imports
Travoprost is classified as a prescription ophthalmic drug in both the US and European Union. This means that the active pharmaceutical ingredient (API) must meet stringent quality and compliance standards:
- Must be manufactured under Good Manufacturing Practices (GMP)
- Must be backed by a valid Drug Master File (DMF) or Active Substance Master File (ASMF)
- Requires batch-level testing and documentation
- Must comply with FDA and EMA import guidelines
2. Key Regulatory Bodies & Documents Required
| Region | Authority | Documentation |
|---|---|---|
| US | FDA (Food & Drug Administration) | DMF (Type II), GMP Certificate, CoA, Labeling, Import License |
| Europe | EMA (European Medicines Agency), National Authorities | ASMF, CEP (if available), GMP Certificate, CoA, Labeling |
Tip: Ensure that your Travoprost API supplier has filed a DMF with the US FDA and/or an ASMF with the EMA. This can simplify product registration for formulators.
3. Good Manufacturing Practices (GMP) Compliance
GMP compliance is the backbone of international pharmaceutical trade. When importing Travoprost API:
- Insist on GMP-certified manufacturers
- Request GMP audit reports or inspection certificates
- Validate the manufacturer’s quality control systems and storage protocols
In both the US and EU, non-GMP products are barred from entering the pharmaceutical supply chain.
4. Drug Master File (DMF) & Active Substance Master File (ASMF)
Your API manufacturer must have:
- DMF (Drug Master File) for the US FDA
- Submit Letter of Authorization (LOA) to reference it in your NDA/ANDA
- ASMF for EMA (Europe)
- Include an Applicant’s Part (for formulators) and Restricted Part (for regulators only)
If your API has a Certificate of Suitability (CEP) from EDQM, it simplifies the approval process across EU nations.
5. Documentation Checklist for Travoprost API Import
Before import, ensure you or your supplier provides:
- GMP Certificate
- Valid DMF (FDA) or ASMF (EMA)
- Certificate of Analysis (CoA)
- Product Specification Sheet
- Safety Data Sheet (SDS)
- Stability Data
- Labeling & Packaging Details
- Import Permits (if applicable)
- Certificate of Origin
6. Customs and Import Protocols
For the US:
- Submit Entry documents (CBP Form 3461)
- Ensure product is listed in FDA’s Import Trade Auxiliary Communication System (ITACS)
- Include FDA product code, manufacturer registration number, and DMF reference
For Europe:
- Ensure ASMF or CEP is registered with appropriate national competent authorities
- Coordinate with your customs broker to meet tariff classification and import license requirements
7. Cold Chain & Storage Considerations
Travoprost is a temperature-sensitive compound. Key guidelines include:
- Store between -20°C to -10°C
- Ship in insulated cold-chain packaging
- Use data loggers to monitor temperature during transit
- Clearly label with temperature control instructions
Improper storage can invalidate CoA and lead to regulatory rejection.
8. Choosing the Right Travoprost API Supplier
When selecting a supplier, look for:
- GMP certification and audit reports
- DMF/ASMF availability
- Experience exporting to US/EU markets
- Transparency in batch traceability and documentation
- Strong packaging and logistics capabilities (cold-chain included)
Chemignition Laboratory: Your Travoprost API Import Partner
Chemignition Laboratory manufactures and exports Travoprost API with full regulatory support for both global markets.
What We Provide:
- GMP certified API production
- CoA, MSDS, impurity profile, TSE/BSE declaration
- Stability data
- Customs-compliant packaging and export documentation
- Global shipping (air/sea, temperature-controlled options)
Conclusion
Importing Travoprost API to the US and Europe requires strategic planning and rigorous documentation. By partnering with a GMP-certified, export-compliant manufacturer, ensuring valid DMF or ASMF filings, and following proper customs protocols, you can ensure smooth import, faster formulation approvals, and long-term regulatory compliance.
Looking for a trusted Travoprost API supplier?
Chemignition Laboratory is a GMP-certified manufacturer, supplier, and exporter of Travoprost API—fully compliant with FDA and EMA documentation needs.
📩 Contact us to request COA and pricing details.
FAQs
What is the regulatory requirement to import Travoprost API to the US?
To import Travoprost API into the US, the product must be manufactured under GMP-compliant conditions and supported by a valid DMF (Type II) filed with the US FDA. Importers also need to provide documentation like Certificate of Analysis (CoA), GMP certificate, and import permits.
Is a DMF mandatory for Travoprost API registration in the US?
Yes, a Drug Master File (DMF) is typically required for Travoprost API when submitting a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) in the US. A Letter of Authorization (LOA) must be submitted to link your application with the DMF.
How can I import Travoprost API into Europe?
To import Travoprost API into Europe, you must source it from a GMP-certified facility and have either a valid ASMF (Active Substance Master File) or CEP (Certificate of Suitability) approved by the EMA or national competent authorities. Cold chain logistics may also be required.
What is the ideal storage condition for Travoprost API?
Travoprost API should be stored between -20°C to -10°C. It must be transported using cold chain logistics with temperature monitoring to maintain product integrity and compliance.
How can I verify if my Travoprost API supplier is GMP-certified?
Request a valid GMP certificate issued by regulatory authorities (such as US FDA, WHO, EU GMP). You can also ask for audit reports or third-party inspection records for additional verification.