Travoprost API is an essential ingredient in ophthalmic drugs used to treat glaucoma and ocular hypertension. Its sensitive nature and regulatory complexity make sourcing Travoprost API a task that requires precision, experience, and vigilance.
However, many pharmaceutical buyers unknowingly make sourcing errors that lead to delays, regulatory setbacks, or compromised product quality. This guide outlines the most common Travoprost API sourcing mistakes and offers actionable strategies to avoid them.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
1. Ignoring Regulatory Compliance and Certifications
❌ Mistake:
Working with suppliers who lack GMP certification or necessary regulatory filings (such as US DMF, EU CEP).
✅ How to Avoid:
- Always request GMP certification, CoA, and regulatory filings.
- Confirm the API is listed with DMF in the U.S. or CEP in Europe.
- Choose suppliers experienced in regulated markets.
2. Focusing Only on Price
❌ Mistake:
Selecting suppliers based on the lowest quote while ignoring quality assurance, logistics capability, or documentation readiness.
✅ How to Avoid:
- Evaluate total cost of ownership (TCO), including testing, revalidation, customs handling, and logistics.
- Check for hidden costs such as repackaging or delayed shipments.
- Balance cost with supplier reliability.
3. Overlooking Cold Chain Logistics
❌ Mistake:
Failing to verify if the supplier uses validated cold chain logistics suitable for temperature-sensitive APIs like Travoprost.
✅ How to Avoid:
- Ensure API is packed and shipped at -20°C to -10°C with temperature monitoring.
- Ask for shipping validation reports and use GDP-compliant logistics providers.
4. Incomplete or Inaccurate Documentation
❌ Mistake:
Accepting shipments without verifying documentation such as CoA, MSDS, stability data, and BSE/TSE declarations.
✅ How to Avoid:
- Require a complete documentation dossier before purchase.
- Use a compliance checklist for every order.
- Insist on documents that match your regulatory jurisdiction.
5. Not Conducting Supplier Audits
❌ Mistake:
Skipping on-site or virtual audits before engaging with a new API manufacturer.
✅ How to Avoid:
- Perform a virtual or third-party audit of facilities.
- Evaluate their QA/QC systems, batch history, and regulatory inspection track record.
6. Misunderstanding Shelf Life and Storage Conditions
❌ Mistake:
Assuming all API shipments have the same storage requirements or shelf life.
✅ How to Avoid:
- Request stability data.
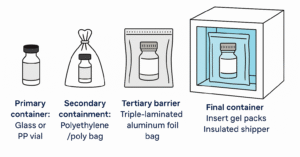
- Confirm packaging standards (e.g., triple-laminated aluminum bag, HDPE container).
- Understand the retest period and storage instructions (-20°C to -10°C).
7. Not Checking Batch Consistency
❌ Mistake:
Assuming one compliant batch means all future batches will meet the same standards.
✅ How to Avoid:
- Ask for batch-specific CoAs.
- Conduct in-house analytical testing or third-party verification.
- Keep records of lot-to-lot consistency.
8. Choosing Traders Instead of Direct Manufacturers
❌ Mistake:
Sourcing from middlemen or traders without transparency into the actual manufacturing site.
✅ How to Avoid:
- Prefer GMP-certified direct manufacturers.
- Ask for site address, plant registration, and manufacturing flow diagram.
9. Ignoring Supply Chain Risk Factors
❌ Mistake:
Failing to assess geopolitical, environmental, or logistical risks in supplier regions.
✅ How to Avoid:
- Develop a risk mitigation plan.
- Maintain multiple supplier options.
- Consider supplier locations with fewer regulatory restrictions or export barriers.
10. Poor Communication and Coordination
❌ Mistake:
Delayed communication can lead to missed shipment windows or misinterpretation of specs.
✅ How to Avoid:
- Establish clear communication protocols.
- Assign dedicated project coordinators or sourcing managers.
- Use digital tools for order tracking and document exchange.
Final Thoughts
Travoprost API sourcing mistakes is not just a procurement task—it’s a high-stakes process that directly impacts drug quality, compliance, and patient safety.
Avoiding these common mistakes can help pharmaceutical companies reduce delays, avoid regulatory penalties, and ensure a stable supply of high-quality API.
Why Choose Chemignition Laboratory?
Chemignition Laboratory is a GMP-certified manufacturer and exporter of ophthalmic APIs, including Travoprost and Latanoprost. We provide:
- Complete regulatory documentation
- Cold chain logistics
- Batch consistency assurance
- Direct manufacturer transparency
- Custom audits on request
Ready to source Travoprost API the right way?
Website: www.chemignition.com
FAQs
What are the most common Travoprost API sourcing mistakes?
The most common mistakes include choosing suppliers without GMP certification, focusing only on price, neglecting cold-chain logistics, failing to audit suppliers, and sourcing through non-transparent traders.
Why is GMP certification important when sourcing Travoprost API?
GMP certification ensures the API is manufactured under controlled and compliant conditions, reducing the risk of contamination, inconsistency, or regulatory rejection during product registration or audits.
How does poor documentation affect Travoprost API procurement?
Incomplete or incorrect documentation (like CoA, MSDS, or stability data) can delay regulatory approvals, result in customs clearance issues, or lead to product recalls. Always verify documents before purchase.
What storage conditions should I expect for Travoprost API?
Travoprost API should be stored between -20°C to -10°C in light-resistant, airtight packaging such as a triple-laminated aluminum bag inside an HDPE container. Failure to meet these standards can degrade product quality.
Should I source Travoprost API through a trader or directly from the manufacturer?
It’s recommended to work directly with GMP-certified manufacturers to ensure transparency, better pricing, faster resolution of quality issues, and full traceability.
Can I rely on a low-cost supplier for Travoprost API?
Not always. Low-cost suppliers may compromise on documentation, stability testing, packaging quality, or delivery reliability. Always weigh price against regulatory compliance and long-term reliability.
What should be included in a proper Travoprost API documentation set?
A compliant set should include:
Certificate of Analysis (CoA)
MSDS/SDS
Stability data
BSE/TSE certificate
GMP certificate
Regulatory filings (US DMF, EU CEP if applicable)