What is GMP Tafluprost?
Tafluprost API is a prostaglandin analog used in ophthalmic formulations to treat glaucoma and ocular hypertension. It works by reducing intraocular pressure (IOP) through increased aqueous humor outflow, helping to protect the optic nerve from damage and prevent vision loss.
Chemignition Laboratory is a leading manufacturer and exporter of GMP-certified Tafluprost API worldwide. They adhere to strict Quality Management Systems (QMS) to maintain compliance with Good Manufacturing Practices (GMP). Their commitment to quality and regulatory standards ensures that Tafluprost API is highly pure, safe, and effective for ophthalmic formulations.
Properties of GMP Tafluprost
- Product Name: Tafluprost
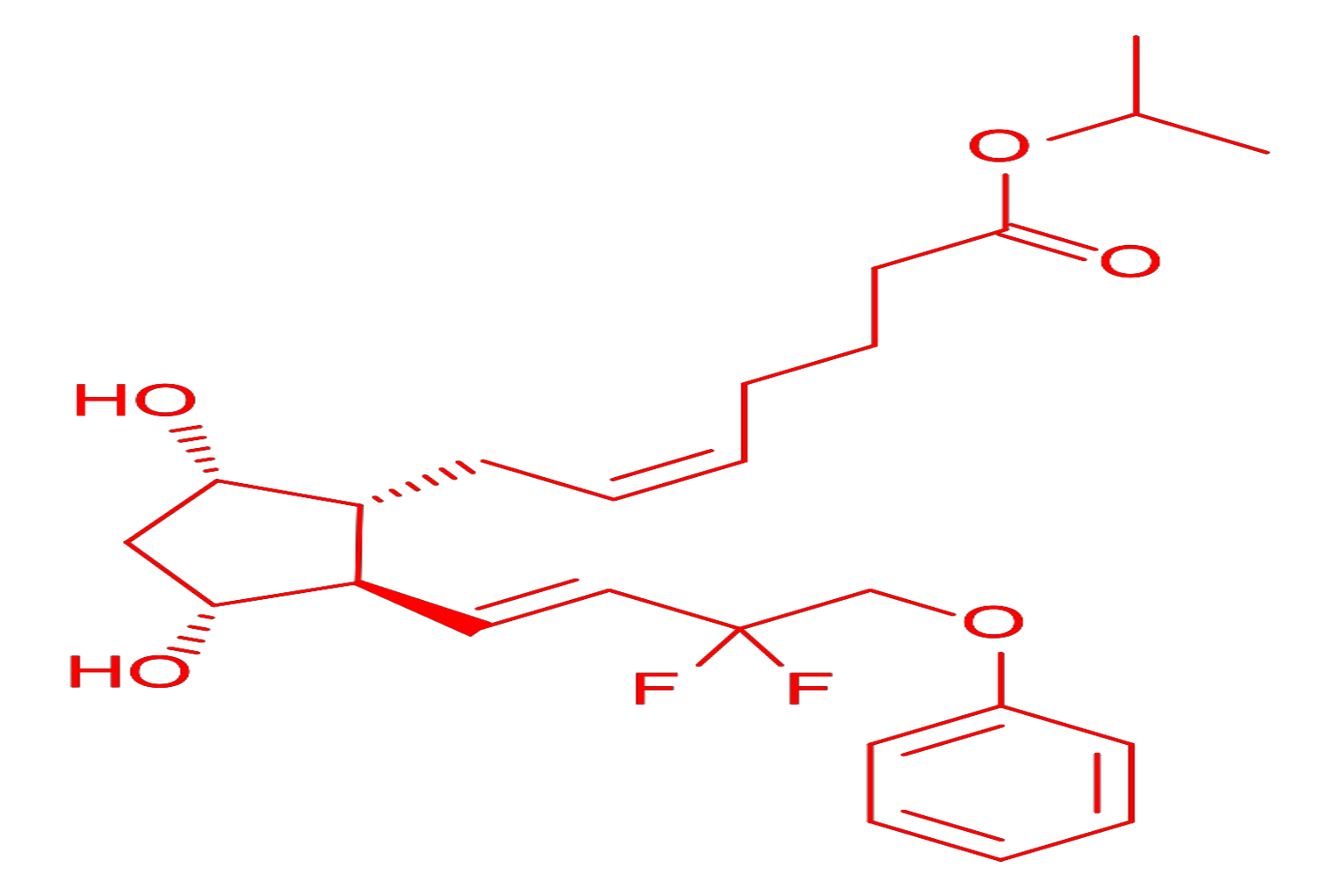
- IUPAC Name: Isopropyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-phenoxybut-1-en-1-yl]-3,5-dihydroxycyclopentyl}hept-5-enoate
- CAS No: 209860-87-7
- Chemical Formula: C₂₅H₃₄F₂O₅
- Grade: USP/IP/IH
- Appearance: Colorless to pale yellow liquid.
- Molar Mass: 452.6 g/mol
- Solubility: Insoluble in water but soluble in organic solvents such as ethanol and acetonitrile.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
Applications of GMP Tafluprost
Since Tafluprost API is primarily used in ophthalmology, it has multiple key applications, including:
- Glaucoma treatment – Reduces intraocular pressure (IOP) and prevents optic nerve damage
- Ocular hypertension therapy – Helps maintain healthy eye pressure levels
- Preservative-free formulations – Tafluprost is often used in preservative-free eye drops to reduce irritation in sensitive patients
As a result, Tafluprost API is in high demand among eye drop manufacturers and ophthalmic pharmaceutical companies.
Related Products
Key Considerations for API Buyers & Manufacturers
Before purchasing GMP Tafluprost, API buyers and ophthalmic formulation companies should evaluate the following factors:
1️⃣ Supplier GMP Certification – Ensure that the supplier meets international GMP standards
2️⃣ Purity & Quality Standards – Verify impurity levels, stability, and sterility
3️⃣ Regulatory Approvals – Confirm compliance with global health authorities
4️⃣ Batch Consistency – Maintain uniform formulation to ensure consistent patient outcomes
By carefully considering these factors, manufacturers can ensure product quality and regulatory compliance, leading to safer and more effective ophthalmic formulations.
How to Order GMP Tafluprost
GMP Tafluprost plays a critical role in ophthalmic pharmaceuticals, ensuring that glaucoma and ocular hypertension treatments remain safe and effective.
Pharma API buyers and eye drop manufacturers recognize the importance of sourcing from a GMP-certified supplier. Furthermore, working with a trusted and experienced company ensures consistent quality and regulatory compliance. For this reason, Chemignition Laboratory serves as an essential partner in maintaining high-quality formulations.
If you’re looking for GMP Tafluprost, reach out to Chemignition Laboratory today! 🚀
FAQs
Tafluprost API manufacturer india?
Chemignition laboratory is leading manufacturer and supplier of Tafluprost API.
What is the CAS number of Tafluprost?
The CAS number for Tafluprost is 209860-87-7.
How does Tafluprost work for glaucoma and ocular hypertension?
Tafluprost increases the outflow of aqueous humor, reducing intraocular pressure (IOP), which helps protect the optic nerve from damage.
Who should buy GMP Tafluprost API?
Pharmaceutical API buyers, ophthalmic formulation manufacturers, and eye drop producers should source GMP-certified Tafluprost to ensure regulatory compliance and product quality.
Where can I source high-quality GMP Tafluprost API?
Chemignition Laboratory is a trusted manufacturer and exporter of GMP-certified Tafluprost API, ensuring high purity, batch consistency, and regulatory compliance.