In the highly specialized world of ophthalmic pharmaceuticals, choosing the right Active Pharmaceutical Ingredient (API) supplier is more than a procurement decision—it’s a matter of product success, regulatory compliance, and patient safety. Among the key ingredients used to manage glaucoma and ocular hypertension, Tafluprost API stands out due to its potency, stability requirements, and clinical efficacy.
This blog explores the role of Tafluprost API, its applications, and why Chemignition Laboratory is the go-to manufacturer, global supplier, and exporter for pharmaceutical companies worldwide.
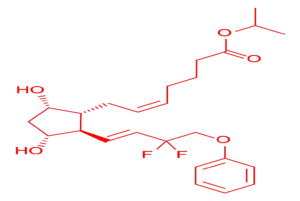
What is Tafluprost API?
Tafluprost is a prostaglandin analog that mimics the natural prostaglandin F2α, reducing intraocular pressure (IOP) in the eye by increasing the uveoscleral outflow of aqueous humor. Tafluprost is primarily used in ophthalmic formulations like eye drops, helping manage conditions such as:
- Open-angle glaucoma
- Ocular hypertension
- Post-operative IOP elevation
The Active Pharmaceutical Ingredient (API) form of Tafluprost is the base compound used by formulation companies to develop finished pharmaceutical products (FPPs) that are dispensed to patients.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
Importance of API Quality in Ophthalmic Formulations
Ophthalmic APIs like Tafluprost must meet rigorous standards for sterility, purity, stability, and biocompatibility. Any variation in the quality of the API can compromise the safety and effectiveness of the final product.
Chemignition Laboratory ensures that every batch of Tafluprost API manufactured complies with the highest international standards, including:
- IP (Indian Pharmacopoeia)
- BP (British Pharmacopoeia)
- EP (European Pharmacopoeia)
- USP (United States Pharmacopeia)
Our commitment to consistent quality and regulatory adherence has made us a preferred Tafluprost API supplier in India and across the globe.
Chemignition Laboratory: Who We Are
Chemignition Laboratory is a GMP-certified manufacturer of high-purity APIs, specializing in ophthalmic, cardiovascular, and dermatological segments. Based in India, we’ve built a global reputation as a reliable exporter of Active Pharmaceutical Ingredients, serving companies in the United States, Europe, Southeast Asia, and Latin America.
We combine state-of-the-art infrastructure, strict quality protocols, and a customer-first approach to become a trusted long-term partner for pharmaceutical companies.
Technical Specifications of Tafluprost API
| Parameter | Details |
|---|---|
| Product Name | Tafluprost |
| CAS Number | 209860-87-7 |
| Molecular Formula | C25H34F2O5 |
| Appearance | White to off-white powder |
| Grade Available | IP / BP / USP / EP |
| Assay (By HPLC) | ≥ 99% |
| Storage Condition | –20°C to –10°C, light-resistant |
| Packaging | Glass/polypropylene vials, triple-laminated aluminum pouches, HDPE containers |
All our Tafluprost APIs come with a Certificate of Analysis (COA), Material Safety Data Sheet (MSDS), and batch-specific documentation.
Global Export Capabilities
As a leading Tafluprost API exporter, Chemignition Laboratory supports clients around the world. We understand the complex logistics and documentation required to export APIs to regulated markets like:
- USA (FDA registered agents supported)
- Europe (EMA & EUGMP standards)
- Middle East and North Africa (MENA region)
- Southeast Asia & Oceania
We assist clients with:
- Import/export registration support
- Cold-chain shipping for stability-sensitive APIs
- Custom packaging and labeling based on client needs
Why Choose Chemignition for Tafluprost API?
✅ 1. GMP-Certified Manufacturing
Our facility operates under Good Manufacturing Practice (GMP) guidelines, ensuring product consistency, safety, and compliance with global standards.
✅ 2. End-to-End Traceability
We offer complete traceability from raw material procurement to final API dispatch, backed by thorough documentation.
✅ 3. High Batch Consistency
We ensure every lot of Tafluprost API matches the specifications of the previous batch, maintaining quality for scale-up and formulation development.
✅ 4. Regulatory Compliance
Whether you’re filing with other health authorities, our APIs are supported by:
- Analytical validation
- Stability studies
- Residual solvent profile
✅ 5. Flexible Order Quantities
Whether you need a few grams for R&D or multiple kilograms for commercial production, we can fulfill your requirements efficiently.
✅ 6. Dedicated Support Team
Our technical and export teams provide real-time assistance with:
- COA and MSDS issuance
- Regulatory query resolution
- Logistics and shipping coordination
Applications of Tafluprost API
Tafluprost API is typically formulated into topical ophthalmic solutions with or without preservatives. Its primary benefits include:
- Effective IOP reduction in patients non-responsive to beta-blockers
- Lower systemic side effects due to localized action
- Compatibility with other ocular APIs like timolol or latanoprost in fixed-dose combinations
It is used in both preservative-free single-dose packaging and multi-dose bottle formulations.
Packaging & Storage Best Practices
To maintain stability and shelf-life, Tafluprost API from Chemignition is packaged using a 3-tier protocol:
- Primary Packaging: Type I glass or polypropylene vial
- Secondary Packaging: High-density polybag secured with a PVC strip
- Tertiary Packaging: Triple-laminated aluminum pouch placed in an HDPE container
We recommend storage between –20°C to –10°C in a tightly sealed, light-resistant container to avoid degradation.
Certifications & Documentation
We provide complete regulatory support including:
- GMP Certificate
- Certificate of Analysis (COA)
- MSDS
- Technical Dossier
- Stability Data (on request)
Our facility undergoes regular third-party audits and follows ICH Q7 guidelines for API manufacturing.
Bulk Orders & Custom Solutions
We serve a wide range of clients—from early-stage pharmaceutical developers to commercial-scale manufacturers. Depending on your needs, we offer:
- Bulk supply of Tafluprost API
- Pilot batch production
- Custom purity or micronization levels
- Contract manufacturing partnerships
Our scalability makes us ideal for both small-batch development and large-scale procurement.
Trusted by Global Partners
With clients across 30+ countries, Chemignition Laboratory has built a reputation for:
- On-time delivery
- Responsive support
- High product reliability
We’ve helped pharmaceutical brands meet their R&D timelines, launch ophthalmic products successfully, and maintain competitive edge in regulated markets.
Let’s Work Together
If you’re searching for a Tafluprost API manufacturer or global supplier, choose a partner who prioritizes quality, compliance, and customer success. At Chemignition Laboratory, we help you build reliable, effective formulations backed by world-class API production.
Request a Free Quote or Sample Today
- Email: info@chemignition.com
- Website: www.chemignition.com
- Head Office: Gujarat, India
- Export Support: US, EU, LATAM, Asia
Final Thoughts
In today’s competitive pharmaceutical landscape, sourcing from a trusted Tafluprost API manufacturer can significantly impact your product’s regulatory approval, efficacy, and market success. Choose Chemignition Laboratory for quality you can trust, documentation you can rely on, and a partnership built to last.
FAQs
What is Tafluprost API used for?
Tafluprost API is used in the manufacturing of ophthalmic formulations, primarily eye drops for treating open-angle glaucoma and ocular hypertension. It works by reducing intraocular pressure through increased aqueous humor outflow.
Who is Manufacturer and exporter of Tafluprost API?
Chemignition Laboratory is trusted manufacturer and exporter of Tafluprost API.
Who is the best manufacturer of Tafluprost API?
Chemignition Laboratory is one of the most trusted Tafluprost API manufacturers, known for GMP-certified production, global exports, and high-purity ophthalmic-grade APIs.
Is Tafluprost API available in IP, BP, USP, and EP grades?
Yes. Chemignition Laboratory offers Tafluprost API in multiple grades including IP, BP, USP, and EP to meet different regulatory and formulation requirements.
What are the storage conditions for Tafluprost API?
Tafluprost API must be stored at –20°C to –10°C in a tightly sealed, light-resistant container. Proper cold-chain logistics are essential to maintain stability during shipping.
Do you provide regulatory documentation for Tafluprost API?
Yes. We provide complete documentation such as GMP certificate, COA, MSDS and MOA (on request) to comply with global regulatory requirements.