Glaucoma remains one of the world’s leading causes of irreversible blindness, impacting over 76 million people globally. Effective management relies heavily on topical prostaglandin analogues, among which Travoprost API for Glaucoma plays a critical role.
This blog explores the importance of the Travoprost API for Glaucoma, focusing on its clinical utility, advantages over alternatives, market outlook, and key sourcing considerations for buyers and pharmaceutical manufacturers.
✅ Chemignition Laboratory is a GMP-certified manufacturer and global exporter of Travoprost API, delivering regulatory-compliant material for both regulated and semi-regulated markets.
1. What Is Travoprost API?
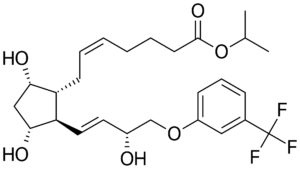
Travoprost is a synthetic prostaglandin F2α analogue used in ophthalmic formulations to reduce intraocular pressure (IOP) in patients with open-angle glaucoma and ocular hypertension.
- Molecular Formula: C26H35F3O6
- CAS Number: 157283-68-6
- Drug Class: Prostaglandin analogue
It works by increasing uveoscleral outflow of aqueous humor from the eye, thereby lowering IOP and reducing optic nerve damage over time.
2. Uses of Travoprost API in Ophthalmology
Travoprost is formulated into eye drops, typically at 0.004% concentration, and prescribed once daily. Its primary applications include:
- Open-angle glaucoma (most common form)
- Ocular hypertension
- Normal-tension glaucoma (in select cases)
- Adjunct therapy with beta-blockers
🔹 It is favored due to its once-daily dosing, high efficacy, and fewer systemic side effects compared to beta-blockers or carbonic anhydrase inhibitors.
3. Benefits of Travoprost API Over Other Glaucoma Treatments
| Feature | Travoprost | Timolol (Beta-blocker) | Latanoprost/Bimatoprost |
|---|---|---|---|
| Mechanism | Prostaglandin analogue | Reduces aqueous humor production | Prostaglandin analogue |
| Dosing Frequency | Once daily | Twice daily | Once daily |
| Systemic Side Effects | Minimal | Moderate (cardiac, pulmonary) | Mild |
| Ocular Tolerability | Good | Good | Varies |
| Preservative-Free Option | Available | Rare | Available |
🔹 Travoprost API for Glaucoma offers:
- Strong IOP-lowering effect (28–31%)
- Good patient compliance due to once-daily use
- Lower systemic risk compared to older therapies
4. Global Market Demand for Travoprost API
The demand for Travoprost API for Glaucoma is rising due to:
- Growing geriatric population with increased glaucoma incidence
- Shift toward prostaglandin analogues as first-line treatment
- Wider adoption in Asia-Pacific and LATAM markets
- Demand for preservative-free and fixed-dose combinations (Travoprost + Timolol)
📈 Market Forecast:
- Global ophthalmic drugs market: $35B+ by 2028
- Prostaglandin analogue market share: 45%+
- Travoprost API CAGR: ~5.2% (2023–2028)
5. Travoprost API Regulatory Profile
To enter regulated markets, Travoprost API must comply with:
- WHO-GMP manufacturing
- US FDA DMF (Type II)
- EU ASMF or CEP submission
- Stability and impurity profiling
- TSE/BSE-free declarations
Chemignition Laboratory provides complete regulatory documentation for Travoprost API:
- GMP certificate, CoA and MSDS
- Impurity profile and stability data
- Cold chain validation and shelf-life report
6. Storage & Packaging Requirements
Travoprost API is chemically sensitive and requires:
- Storage at -20°C to -10°C
- Light-resistant, tightly sealed containers
- Primary packaging in glass/PP vials, sealed in poly bags, foil pouches
- HDPE outer container with gel packs inside insulated shippers
Shelf life: Up to 36 months when stored correctly
Chemignition Laboratory uses validated cold-chain logistics and temperature tracking for all exports.
7. Travoprost API Buyer Checklist
Before purchasing Travoprost API for Glaucoma formulations, ensure your supplier provides:
- GMP certification
- DMF/ASMF access
- CoA + impurity profile
- Cold-chain packaging declaration
- Shelf-life documentation
- Technical support for audits/filings
🔼 Avoid sourcing from unverified suppliers, especially for ophthalmic actives used in regulated products.
Why Choose Chemignition for Travoprost API?
- GMP certified API facility
- 36-month shelf life with validated stability
- Regulatory support
- Cold-chain compliant packaging & tracking
- Global shipping to 25+ countries
- Flexible MOQs for R&D and commercial use
🔹 Whether you’re formulating generic eye drops or combination therapies, Chemignition delivers high-purity Travoprost API for Glaucoma with documentation and reliability you can trust.
Conclusion
Travoprost API for Glaucoma is a trusted solution for IOP control, offering significant therapeutic benefits, excellent tolerability, and increasing global market demand.
As the need for ophthalmic care grows, sourcing GMP-certified Travoprost API with regulatory documentation, validated storage, and proven supply reliability becomes essential.
Chemignition Laboratory is your strategic partner for high-quality, globally compliant Travoprost API.
📢 Ready to source Travoprost API for Glaucoma?
📩 Contact Chemignition Laboratory for pricing, samples, or regulatory documents.
FAQs
What is Travoprost API used for?
Travoprost API is primarily used to manufacture ophthalmic eye drops for the treatment of open-angle glaucoma and ocular hypertension. It helps reduce intraocular pressure (IOP) by increasing the outflow of aqueous humor from the eye.
How does Travoprost compare to other glaucoma treatments?
Travoprost is a prostaglandin analogue that provides strong IOP-lowering effects with once-daily dosing. Compared to beta-blockers like Timolol, it has fewer systemic side effects and better patient compliance.
What are the regulatory requirements for Travoprost API?
To be accepted in regulated markets, Travoprost API must come from a GMP-certified facility and be supported by DMF (Drug Master File), ASMF, CoA, impurity profiles, and validated stability data.
What storage conditions are required for Travoprost API?
Travoprost must be stored at −20°C to −10°C in light-resistant, airtight packaging. Cold-chain shipping with validated gel packs and insulated containers is essential to maintain its potency.
Why choose Chemignition as a Travoprost API supplier?
Chemignition Laboratory offers GMP certified Travoprost API with full regulatory documentation, a 36-month shelf life, cold-chain shipping, and global delivery support to over 25 countries.