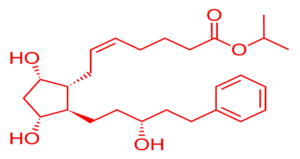
When it comes to formulating effective treatments for glaucoma, ocular hypertension, or even cosmetic eyelash growth, two active pharmaceutical ingredients (APIs) lead the way: Latanoprost vs Bimatoprost.
Although both belong to the prostaglandin analogue class, their mechanism of action, chemical structure, and formulation behavior differ in meaningful ways.
Chemignition Laboratory is a GMP-certified manufacturer, supplier, and global exporter of both Latanoprost and Bimatoprost active pharmaceutical ingredients (APIs).
In this blog, we break down the key differences between the two APIs so that formulators, regulatory teams, and product developers can make informed sourcing and formulation decisions.
1. Chemical Classification & Structure
| Feature | Latanoprost | Bimatoprost |
|---|---|---|
| Class | Prostaglandin F2α analogue | Prostamide analogue |
| Molecular Formula | C26H40O5 | C25H37NO4 |
| Solubility | Insoluble in water, but it is soluble in organic solvents such as ethanol and methanol. | Practically insoluble in water, but it is soluble in organic solvents such as ethanol and methanol |
| CAS Number | 130209-82-4 | 155206-00-1 |
➡ Formulation Insight: Bimatoprost is structurally unique because it acts on a different receptor group, which gives it a dual mechanism of action and influences its stability profile.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
2. Mechanism of Action
| API | Action |
|---|---|
| Latanoprost | Increases uveoscleral outflow |
| Bimatoprost | Increases both uveoscleral and trabecular outflow (dual pathway) |
✅ Result: Bimatoprost is often considered more effective in reducing intraocular pressure (IOP), especially in patients unresponsive to Latanoprost.
3. Efficacy in Glaucoma and Ocular Hypertension
| Feature | Latanoprost | Bimatoprost |
|---|---|---|
| Onset of Action | 3–5 hours | 4 hours |
| Peak IOP Reduction | ~25–30% | ~28–33% |
| Duration of Effect | 24 hours | 24 hours |
📌 Clinical studies show that Bimatoprost may offer 1–2 mmHg more IOP reduction compared to Latanoprost in some patient groups.
4. Tolerability and Side Effect Profile
| Side Effect | Latanoprost | Bimatoprost |
|---|---|---|
| Conjunctival hyperemia | Less frequent | More common |
| Eyelid pigmentation | Rare | More common |
| Eyelash growth | Minimal | Pronounced — often a desired effect |
| Iris pigmentation | Present in both | Present in both |
💡 For formulators of cosmetic eyelash serums, Bimatoprost is preferred due to its clinically proven lash-enhancing effect.
5. Cosmetic vs. Ophthalmic Use
| Application Area | Latanoprost | Bimatoprost |
|---|---|---|
| Approved for Glaucoma | ✅ Yes | ✅ Yes |
| Used in Lash Serums | ❌ Rare | ✅ Widely used (e.g., Latisse®) |
| Cosmetic Licensing Available | ❌ Limited | ✅ Available (INCI data, REACH) |
📢 Chemignition provides both pharma-grade and cosmetic-grade Bimatoprost API along with INCI documentation and regulatory support for global exports.
6. Storage, Stability & Packaging
| Parameter | Latanoprost | Bimatoprost |
|---|---|---|
| Storage Before Opening | Refrigerated (-20°C to -10°C) | Refrigerated (-20°C to -10°CC) |
| Light Sensitivity | High | Very High |
| Shelf Life (Sealed) | ~36 months | ~36 months |
| Shipping Needs | Cold chain preferred | Cold chain preferred |
7. Regulatory Documentation Requirements
| Document | Latanoprost | Bimatoprost |
|---|---|---|
| US FDA Type II DMF | ✅ Available | ✅ Available |
| EU ASMF or CEP | ✅ Available | ✅ Available |
| WHO-GMP Certificate | ✅ Yes | ✅ Yes |
| MSDS, CoA, Impurity Profile | ✅ Complete | ✅ Complete |
👉 Chemignition Laboratory provides a complete documentation pack for both APIs including:
- GMP certificate
- Certificate of Analysis (CoA)
- MSDS
- TSE/BSE declaration
- Stability data
- Impurity profile
8. Cost & Market Availability
| Aspect | Latanoprost | Bimatoprost |
|---|---|---|
| API Price (avg/1 g) | $500–$550 | $310–$.350 |
| MOQ | Flexible | Flexible |
| Market Demand | Steady (ophthalmic only) | High (ophthalmic + cosmetic) |
🎯 Bimatoprost offers higher commercial value in the cosmetic sector, justifying its premium pricing.
Why Formulators Choose Chemignition for Latanoprost vs Bimatoprost
At Chemignition Laboratory, we specialize in ophthalmic and cosmetic APIs with global regulatory readiness.
We offer:
- GMP certified manufacturing
- Documents support
- Trial batch availability
- Export support to regulated and semi-regulated markets
- Batch customization, small MOQs, and global logistics handling
Whether you’re building a glaucoma generic, an eye care line, or a beauty brand, we support your journey from sourcing to compliance.
Conclusion
Both Latanoprost vs Bimatoprost are valuable APIs in the ophthalmic space — each with unique benefits:
- Choose Latanoprost for cost-effective glaucoma treatments with high patient tolerance.
- Choose Bimatoprost for dual-action IOP reduction and cosmetic benefits like eyelash growth.
Chemignition Laboratory is your trusted manufacturer, supplier, and exporter for Latanoprost and Bimatoprost API, with full compliance support, flexible supply terms, and global reach.
📢 Ready to source GMP-certified Latanoprost or Bimatoprost?
📩 Contact Chemignition Laboratory now for a quote, DMF access, or documentation pack.
FAQs
What’s the main difference between Latanoprost and Bimatoprost?
Latanoprost is a prostaglandin F2α analogue that increases uveoscleral outflow, while Bimatoprost is a prostamide that boosts both uveoscleral and trabecular outflow, often resulting in greater intraocular pressure (IOP) reduction.
Which API is more effective in treating glaucoma?
Clinical studies suggest that Bimatoprost may offer slightly superior IOP reduction compared to Latanoprost, making it suitable for patients with advanced glaucoma or poor response to other drugs.
Can Bimatoprost be used in cosmetic formulations?
Yes. Bimatoprost is approved for cosmetic eyelash growth applications and is widely used in serums. Latanoprost has no such cosmetic use approval.
Is there a difference in storage and shipping requirements?
Yes. Latanoprost and Bimatoprost generally requires refrigeration (-20°C to -10°C).
Does Chemignition supply both APIs with regulatory documentation?
Absolutely. Chemignition Laboratory provides GMP-certified Latanoprost and Bimatoprost API with CoA, MSDS, stability data, and impurity profiles — ready for regulated and semi-regulated markets worldwide.