
In the pharmaceutical industry, data integrity plays a crucial role in maintaining product quality, regulatory compliance, and patient safety. Without reliable data, pharmaceutical companies risk compliance failures, legal consequences, and compromised product efficacy. Therefore, organizations must implement stringent measures to ensure data accuracy, completeness, and consistency throughout the manufacturing and quality control processes.
What is Data Integrity?
Data integrity refers to the completeness, consistency, and accuracy of data throughout its lifecycle. It ensures that data remains unchanged, authentic, and available for regulatory review.
Maintaining data integrity is crucial for compliance with:
- Good Manufacturing Practices (GMP) – Ensures consistent production and quality standards.
- Good Laboratory Practices (GLP) – Guarantees the reliability of non-clinical laboratory studies.
- Good Clinical Practices (GCP) – Maintains the credibility and accuracy of clinical trial data.
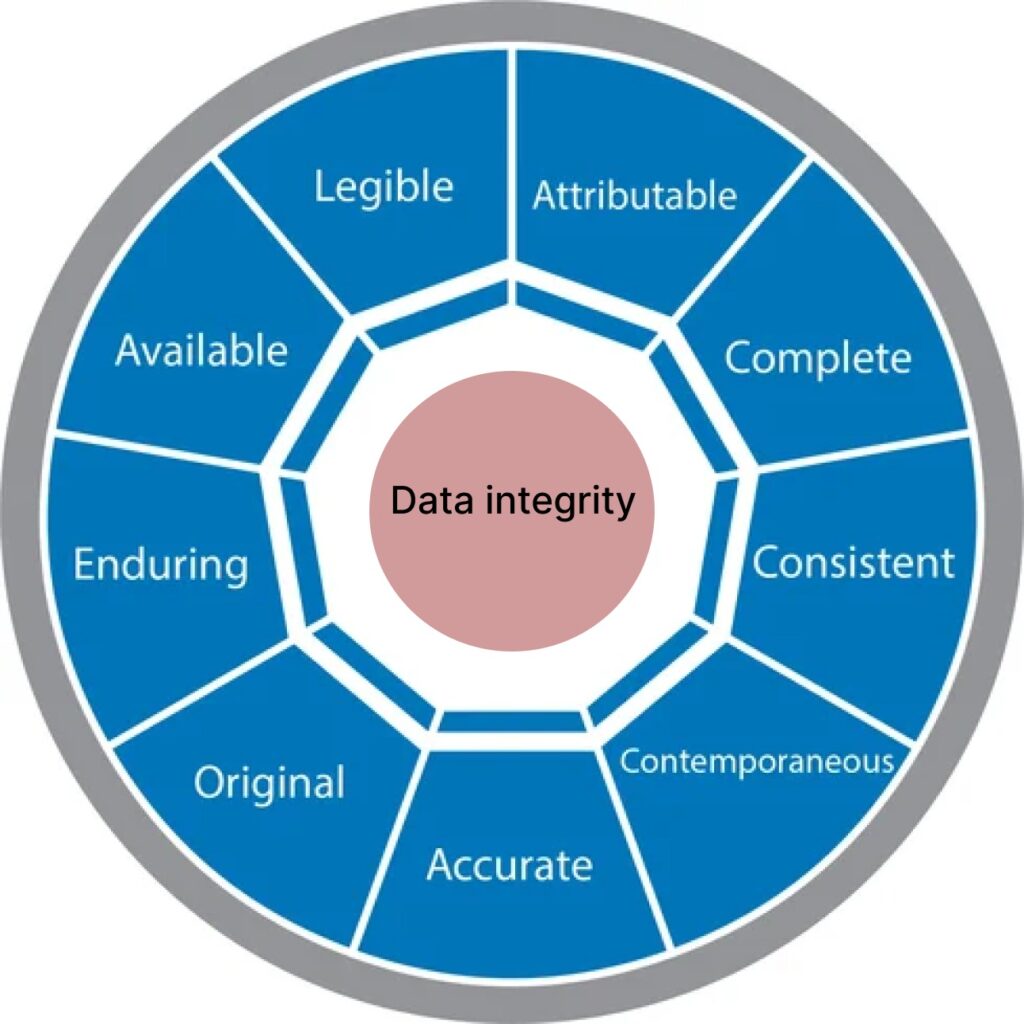
Understanding ALCOA, ALCOA+, and ALCOA++
The ALCOA principles provide a structured framework for data integrity, ensuring that data is:
- Attributable – Linked to the person or system that generated it.
- Legible – Clear, readable, and permanently recorded.
- Contemporaneous – Recorded at the time it is generated.
- Original – The first recorded observation is maintained.
- Accurate – Correct, valid, and truthful.
ALCOA+: Enhancing Data Integrity
As regulatory expectations evolved, ALCOA was expanded into ALCOA+ by adding four more attributes:
- Complete – Includes all relevant data, including errors and corrections.
- Consistent – Maintains a logical sequence with time and date stamps.
- Enduring – Recorded on durable media.
- Available – Easily retrievable throughout the retention period.
ALCOA++: Strengthening Compliance
ALCOA++ further enhances by incorporating traceability, ensuring that all modifications are documented and audit trails are maintained.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
The Importance of Data Integrity in Pharmaceuticals
Regulatory agencies enforce strict guidelines to ensure pharmaceutical data remains accurate and traceable. Any data manipulation or inconsistency can result in regulatory actions, product recalls, and reputational damage. Additionally, it ensures the reliability of research and development, manufacturing processes, and quality control testing. Consequently, pharmaceutical companies must establish robust systems to prevent data breaches and errors.
Strategies to Maintain Data Integrity
To uphold, pharmaceutical companies should implement the following best practices:
- Automated Systems and Digitalization – Using electronic data capture and validated software reduces human errors and enhances traceability.
- Regular Audits and Monitoring – Conducting internal and external audits helps identify gaps and ensures compliance.
- Training and Awareness Programs – Employees must understand integrity requirements and their impact on compliance.
- Access Controls and User Authentication – Restricting access to authorized personnel prevents unauthorized data modifications.
- Robust Documentation Practices – Accurate record-keeping ensures compliance and provides a clear audit trail.
Challenges in Maintaining Data Integrity
Although pharmaceutical companies strive for data, several challenges may arise, including:
- Human Errors – Manual data entry increases the risk of mistakes.
- System Vulnerabilities – Inadequate cybersecurity measures can lead to data breaches.
- Lack of Awareness – Employees unaware of integrity principles may unknowingly compromise compliance.
By addressing these challenges, organizations can strengthen their data management processes and ensure regulatory adherence.
Conclusion
Data integrity is the backbone of pharmaceutical compliance, ensuring that data remains reliable, traceable, and auditable. By following ALCOA, ALCOA+, and ALCOA++ principles, pharmaceutical companies can enhance compliance, protect patient safety, and maintain industry credibility. As regulatory expectations continue to evolve, prioritizing data integrity will remain essential for success in the pharmaceutical sector.
FAQs
Why is data integrity important in the pharmaceutical industry?
Data integrity ensures the accuracy, reliability, and consistency of data used in drug manufacturing, testing, and compliance. It helps prevent errors, fraud, and regulatory violations while ensuring patient safety.
What are the ALCOA+ principles?
ALCOA+ stands for Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, and Available. These principles ensure data remains trustworthy and compliant with regulatory standards.
What are common challenges in maintaining data integrity?
Challenges include human errors, improper documentation, system vulnerabilities, unauthorized access, and lack of proper training. Implementing strict controls and automated systems can help mitigate these risks.
How can pharmaceutical companies improve data integrity?
Companies can enhance data integrity by implementing electronic data management systems, training employees, conducting internal audits, and following ALCOA+ principles to ensure accurate record-keeping.
How does data integrity relate to Good Manufacturing Practices (GMP)?
GMP guidelines require accurate and reliable data to ensure consistent product quality. Data integrity violations can result in regulatory action and impact manufacturing operations.



