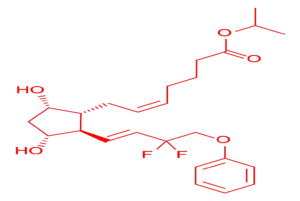
Tafluprost API plays a crucial role in treating glaucoma and ocular hypertension. It works by reducing intraocular pressure, which helps prevent optic nerve damage and vision loss.
Chemignition Laboratory is the leading high-purity Tafluprost Exporter pharmaceutical manufacturers worldwide. By maintaining strict quality control, adhering to global regulatory standards, and ensuring a reliable supply chain, the company provides pharmaceutical-grade Tafluprost that meets international requirements.
About Chemignition Laboratory
Chemignition Laboratory started its journey in 2018 and has since grown into a leading pharmaceutical API exporter. The company specializes in developing, manufacturing, and marketing high-quality intermediates and APIs.
Over the years, it has expanded its product range and now supplies pharmaceutical ingredients to both domestic and international markets. By integrating advanced scientific research, technology, and strategic market intelligence, Chemignition Laboratory stays ahead in the pharmaceutical industry.
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
End-to-End Support
Chemignition Laboratory provides comprehensive support services to help clients through every stage of the product lifecycle. From research and development to commercialization, the company ensures a smooth process for its partners.
Its support services include:
- Conducting research and development (R&D) to improve formulations
- Performing analytical testing to ensure product quality
- Managing intellectual property rights to protect innovations
- Offering market research and strategy development to help businesses grow
- Providing regulatory documentation assistance for smooth approvals
By continuously enhancing these services, Chemignition Laboratory helps clients develop, launch, and manage high-quality pharmaceutical products efficiently.
Why Choose Chemignition Laboratory as Your Tafluprost Exporter?
High-Purity Tafluprost API
Chemignition Laboratory follows strict quality control measures to ensure Tafluprost meets international pharmaceutical standards. Before export, it tests every batch for purity, stability, and effectiveness. This guarantees that pharmaceutical manufacturers receive only the highest quality Tafluprost API.
Global Reach and Reliable Supply Chain
The company exports Tafluprost API to over 60 countries, ensuring a steady and timely supply to its global clients. By maintaining a strong logistics network, it guarantees that shipments arrive on time and in perfect condition.
Compliance with International Standards
Chemignition Laboratory meets global pharmaceutical regulations, including GMP, FDA, and ISO 9001 certifications. These accreditations assure clients that Tafluprost API is safe, effective, and manufactured according to industry best practices.
Customized Formulations
Understanding that each client has unique needs, Chemignition Laboratory offers customized Tafluprost formulations. This flexibility allows pharmaceutical companies to create tailored ophthalmic solutions that match their specific product requirements.
Applications of Tafluprost API
Ophthalmic Solutions
Pharmaceutical companies use Tafluprost API to develop eye drops for glaucoma and ocular hypertension. The active ingredient effectively lowers intraocular pressure, reducing the risk of vision loss.
Research and Development
Scientists and pharmaceutical researchers study Tafluprost in clinical trials and new drug development. Ongoing research focuses on improving drug delivery systems and enhancing ophthalmic treatments.
Quality Control and Storage
Chemignition Laboratory implements strict quality assurance protocols to ensure Tafluprost API remains stable and effective throughout its shelf life. The company stores Tafluprost in tightly sealed, temperature-controlled vials at -20°C to -10°C.
Each shipment includes:
A Certificate of Analysis (COA) for quality verification
Regulatory compliance documents to ensure transparency
A Material Safety Data Sheet (MSDS) with handling and safety instructions
These measures guarantee that clients receive only the highest quality Tafluprost API.
Contact Chemignition Laboratory
For bulk orders, custom formulations, or product inquiries, get in touch with Chemignition Laboratory:
📍 Address: Office No. T-21, Silver Plaza, Near Sardar Patel Complex, GIDC Estate, Ankleshwar-393002, Gujarat, India
📩 Email: info@chemignition.com
🌐 Website: www.chemignition.com
FAQs
What is the purity level of Tafluprost API from Chemignition Laboratory?
Chemignition Laboratory provides Tafluprost API with a purity of 99%+, ensuring that it meets pharmaceutical-grade quality.
Does Chemignition Laboratory export Tafluprost globally?
Yes, the company exports Tafluprost API to over 60 countries, complying with global pharmaceutical regulations.
What industries use Tafluprost API?
Pharmaceutical manufacturers and research laboratories use Tafluprost API for:
- Developing ophthalmic solutions for glaucoma treatment
- Conducting clinical research on new drug formulations
How does Chemignition Laboratory store and ship Tafluprost?
The company stores Tafluprost at -20°C to -10°C in sealed, temperature-controlled vials to maintain product stability. It ensures secure packaging for international shipments to prevent contamination.