Pharmaceutical companies widely use latanoprost API for glaucoma treatment. It lowers intraocular pressure (IOP) in patients with open-angle glaucoma and ocular hypertension. As a prostaglandin analog, it increases the outflow of aqueous humor, reducing the risk of optic nerve damage and vision loss.
This article explains the mechanism, benefits, suppliers, formulations, and market trends of latanoprost API for glaucoma.
What is Latanoprost API for Glaucoma?
Latanoprost API serves as the key ingredient in prescription glaucoma eye drops. It helps formulate ophthalmic solutions that lower IOP.
Key properties of latanoprost API:
- Chemical formula: C26H40O5
- Molecular weight: 432.59 g/mol
- CAS number: 130209-82-4
- Dosage form: Ophthalmic solution (0.005%)
- Standard dosage: One drop daily in the affected eye
How Does Latanoprost API for Glaucoma Work?
Latanoprost API mimics naturally occurring prostaglandins in the eye. After application, it absorbs through the cornea and converts into latanoprost acid, its active form.
Mechanism of action:
- Increases uveoscleral outflow and reduces eye pressure
- Controls IOP for 24 hours with once-daily dosing
- Limits systemic absorption and lowers the risk of side effects
Many healthcare providers prefer latanoprost API over beta-blockers and carbonic anhydrase inhibitors because of its strong IOP-lowering effect and fewer systemic risks.
Benefits of Latanoprost API for Glaucoma Patients
- Lowers IOP by 25-35%, making it a potent treatment
- Requires only once-daily dosing, improving patient compliance
- Produces fewer systemic effects compared to beta-blockers
- Protects long-term vision by preventing optic nerve damage
Possible side effects:
- Causes mild eye redness (conjunctival hyperemia) in some patients
- Gradually darkens the iris color in certain cases
- Stimulates eyelash growth and thickening
- May result in a mild burning sensation or dryness
Despite these side effects, latanoprost remains the first-line treatment for glaucoma because of its safety and effectiveness.
Latanoprost Formulations & Fixed-Combination Therapy
Pharmaceutical companies manufacture latanoprost API as both single-drug eye drops and fixed-dose combinations to enhance its effect.
Latanoprost-Based Eye Drop Formulations
| Brand Name | Active Ingredient | Notes |
|---|---|---|
| Xalatan (Pfizer) | Latanoprost 0.005% | Most prescribed formulation |
| Generic Latanoprost | Latanoprost 0.005% | Available worldwide |
| Xalacom (Pfizer) | Latanoprost + Timolol | Stronger IOP control |
| Fixapost (Santen) | Latanoprost + Timolol | Preservative-free option |
Many healthcare providers prescribe combination therapies like latanoprost + timolol for patients who need greater IOP reduction.
Supplier Comparison: Who Provides the Best Latanoprost API?
Choosing the right API supplier depends on quality, pricing, regulatory approvals, and supply chain reliability. Here is a detailed comparison of the top latanoprost API manufacturers worldwide.
Top Latanoprost API Suppliers Comparison Table
| Supplier | Location | Specialization | Regulatory Compliance |
|---|---|---|---|
| Chemignition Laboratory | India | High-purity latanoprost API | GMP-certified manufacturing |
| Cayman Chemical | USA | Prostaglandin synthesis | Holds CEP certification from EDQM |
| Merck KGaA | Germany | Large-scale ophthalmic API production | Meets global regulatory requirements |
| Pfizer API | USA | In-house API for Xalatan | FDA-approved API supplier |
| LGM Pharma | USA | Bulk API supply for ophthalmic drugs | Provides full regulatory compliance support |
Key Takeaways from Supplier Comparison:
- Chemignition Laboratory offers high-purity latanoprost API at competitive prices.
- Cayman Chemical provides certified API with CEP approval, making it suitable for EU markets.
- Merck KGaA and Pfizer API ensure premium quality and strict regulatory compliance.
- LGM Pharma specializes in bulk API supply, making it ideal for large-scale manufacturers.
Factors to Consider When Choosing a Latanoprost API Supplier
Selecting the right supplier involves evaluating multiple quality and compliance factors.
1. Regulatory Approvals
- Ensure the supplier holds approvals from FDA, EMA, EDQM, or CDSCO for API distribution.
- Look for CEP certification for European market access.
2. Manufacturing Standards & GMP Compliance
- Suppliers must follow Good Manufacturing Practices (GMP) to ensure product purity and stability.
- High-quality suppliers undergo regular quality audits and inspections.
3. Bulk Pricing & Supply Chain Reliability
- Compare bulk pricing between India, USA, and European API manufacturers.
- Evaluate supplier delivery timelines and stock availability.
4. Technical Support & Documentation
- Reputable suppliers provide Certificates of Analysis (CoA), Drug Master Files (DMF), and Stability Data for regulatory submissions.
Market Demand & Growth for Latanoprost API
The demand for latanoprost API continues to rise due to the growing prevalence of glaucoma and the expansion of generic formulations.
Key Market Trends (2024-2030):
- Global glaucoma cases increasing – Projected to reach 112 million by 2040.
- Growth of generic formulations – Cost-effective generic APIs gaining market share.
- Innovation in sustained-release therapies – Research into nanotechnology and implants for better drug delivery.
Latanoprost API Pricing
The cost of latanoprost API depends on factors like purity, manufacturing standards, bulk purchase quantity, and regulatory compliance.
Current Price Range:
- The reference price for latanoprost API stands at $1,266,963 per kilogram, based on industry reports.
- Bulk pricing may vary based on order size, supplier location, and market demand.
- Generic API suppliers from India and China offer more competitive prices than Western suppliers.
Pharmaceutical manufacturers should compare supplier pricing carefully while ensuring compliance with Good Manufacturing Practices (GMP) and regulatory approvals.
Future of Latanoprost API in Glaucoma Treatment
- Researchers are working on sustained-release implants to provide long-term IOP control
- Advanced drug delivery systems like nanotechnology-based eye drops are under development
- More combination therapies will offer better treatment outcomes
Latanoprost API will continue playing a major role in glaucoma treatment as ongoing innovations improve effectiveness and accessibility.
Final Thoughts
Latanoprost API for glaucoma remains a highly effective treatment that delivers strong IOP reduction, minimal side effects, and convenient dosing.
With increasing global demand, major suppliers like Chemignition Laboratory, Cayman Chemical, and Merck KGaA ensure a consistent supply of high-quality latanoprost API.
As researchers develop sustained-release implants, combination therapies, and advanced formulations, latanoprost API will continue leading glaucoma management worldwide.
Why Choose Chemignition for Latanoprost API?
- GMP certified facility
- Full CoA, MSDS, impurity profile with every batch
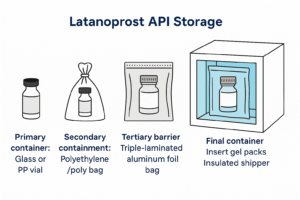
- Shelf life: 36 months with documented stability
- Cold-chain packaging and global delivery
- Technical team support for audits and filings
Chemignition ensures complete transparency from manufacturing to documentation, giving buyers total confidence during regulatory submissions and audits.
FAQs
What is Latanoprost API used for?
Latanoprost API is the active pharmaceutical ingredient in glaucoma eye drops. It helps reduce intraocular pressure (IOP) in patients with open-angle glaucoma and ocular hypertension by increasing aqueous humor outflow.
Which companies supply Latanoprost API?
Major global suppliers of latanoprost API include:
- Chemignition Laboratory (India) – High-purity API manufacturer
- Cayman Chemical (USA) – CEP-certified API supplier
- Merck KGaA (Germany) – Large-scale ophthalmic API production
- Pfizer API (USA) – In-house API supplier for Xalatan
- LGM Pharma (USA) – Bulk API supply for ophthalmic drugs
How do I choose the right Latanoprost API supplier?
To select a reliable API supplier, consider:
- Regulatory approvals (FDA, EMA, EDQM, etc.)
- Manufacturing quality (GMP compliance, stability studies)
- Pricing and bulk order discounts
- Technical support (Certificates of Analysis, Drug Master File availability)