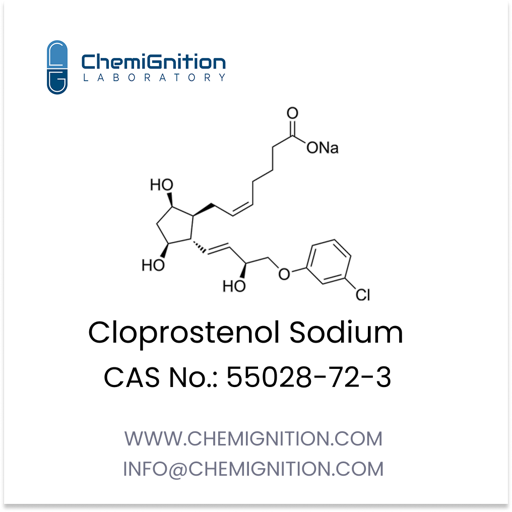
Key Properties of Lubiprostone
| Product Name: | Lubiprostone |
| Synonyms: | 7-[(2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-yl]heptanoic acid |
| CAS No: | 333963-40-9 |
| Chemical Formula: | C20H32F2O5 |
| Grade: | USP/IP/IH |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 390.56 g/mol |
| Solubility: | Insoluble in water but is soluble in organic solvents |
Manufacturing process of Lubiprostone
Before beginning the process operation, check the cleanliness of equipment, confirm the availability of utilities, and verify that all raw materials and packing materials are available.
Charge dichloromethane in glass pot and the starting materials and reagents, including prostaglandin derivatives, are charged. Heat the reaction mass up to 50–60°C and maintain the reaction mass at 50–60°C for 240–360 minutes, Take the in-process sample from reaction mass and send the in-process sample to QC for reaction monitoring by HPLC.
The product is purified using a chromatographic column or liquid-liquid extraction. Crystallization is initiated by cooling the purified solution to 0–5°C and adding an anti-solvent. The product is then filtered and washed with chilled solvent to remove residual impurities. Drying is performed under vacuum at 40–50°C for 480–600 minutes.
Pack Lubiprostone in a glass or polypropylene vial. Place the vial inside a poly bag, twist its mouth, and secure it with a PVC strip. Insert this bag into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Lubiprostone in a tightly closed, light-resistant container at a temperature of 20°C to 25°C.
Applications of Lubiprostone
Technical Specifications of Lubiprostone
| Sr.No. | Test | Specification |
|---|---|---|
| 1 | Description | White to off-white crystalline powder |
| 2 | Solubility | Practically insoluble in water; freely soluble in methanol and ethanol |
| 3 | Identification by IR | The IR spectrum of the sample should be concordant with the reference standard |
| 4 | Assay (on dried basis) | 98.0% to 102.0% |
| 5 | Loss on Drying | Not more than 0.5% when dried at 105°C for 2 hours |
| 6 | Residue on Ignition | Not more than 0.1% |
| 7 | Heavy Metals | Not more than 20 ppm |
| 8 | Related Substances by HPLC | Individual impurity: Not more than 0.1% Total impurities: Not more than 0.5% |
| 9 | Residual Solvents | Complies with USP requirements for residual solvents |
Packing and Storage condition of Lubiprostone
Pack Lubiprostone in a glass or polypropylene vial. Place the vial inside a poly bag, twist its mouth, and secure it with a PVC strip. Insert this bag into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Lubiprostone in a tightly closed, light-resistant container at a temperature of 20°C to 25°C.
MSDS - Lubiprostone
Why Choose Chemignition for
Lubiprostone Supply?
Quality Assurance
Grade Compliance
Lubiprostone is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.
Large-Scale Manufacturing and Export
Grade Compliance
Lubiprostone is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.