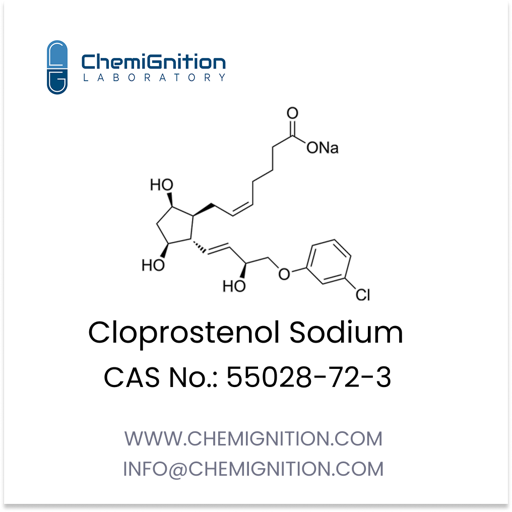
Key Properties of Cloprostenol sodium
| Product Name: | Cloprostenol Sodium |
| Synonyms: | Cloprostenol sodium salt; Prostaglandin F2α analogue |
| CAS No: | 55028-72-3 |
| Chemical Formula: | C22H28Cl2NaO6 |
| Grade: | Veterinary / Pharmaceutical Grade |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 467.84 g/mol |
| Solubility: | Freely soluble in water; soluble in methanol |
Manufacturing process of Cloprostenol sodium
Step 1: Chemical Synthesis
-
The prostaglandin backbone is synthesized through multi-step organic reactions involving stereoselective transformations.
-
Critical steps include:
-
Controlled chain elongation
-
Introduction of halogenated phenyl group
-
Formation of correct stereochemistry (very critical for activity)
-
Step 2: Salt Formation
-
The free acid form of cloprostenol is neutralized with sodium hydroxide or sodium salt reagents
-
This converts it into Cloprostenol Sodium, improving:
-
Water solubility
-
Stability for injectable formulations
-
Step 3: Purification
-
Purification using:
-
Solvent extraction
-
Crystallization
-
Advanced chromatography (if required)
-
-
Impurities are controlled as per veterinary pharmacopeial standards
Step 4: Drying & Milling
-
Vacuum or tray drying under controlled temperature
-
Milling to achieve uniform particle size
Applications of Cloprostenol sodium
Technical Specifications of Cloprostenol sodium
| Sr.No. | Test | Specification |
|---|---|---|
| 01 | Description | White to off-white crystalline powder |
| 02 | Solubility | Freely soluble in water; soluble in methanol |
| 03 | Identification | A) By IR: Infrared absorption spectrum of the sample should correspond to that of the Cloprostenol Sodium reference standard. B) By HPLC: The retention time of the principal peak in the sample chromatogram should match that of the standard preparation. |
| 04 | Water Content | Not more than 5.0% |
| 05 | pH (1% w/v solution) | 7.0 – 8.5 |
| 06 | Related Substances by HPLC | A) Any individual impurity: Not more than 0.5% B) Total impurities: Not more than 2.0% |
| 07 | Assay by HPLC | Not less than 98.0% and not more than 102.0% (on dried basis) |
| 08 | Residual Solvents | Complies with ICH Q3C limits |
Packing and Storage condition of Cloprostenol sodium
Pack Cloprostenol sodium in a glass vial. Place the vial inside a poly bag, twist its mouth, and secure it with a PVC strip. Insert this bag into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Cloprostenol sodium in a tightly closed, light-resistant container at a temperature of 2–8°C.
MSDS - Cloprostenol sodium
Why Choose Chemignition for
Cloprostenol sodium Supply?
Quality Assurance
Grade Compliance
Cloprostenol sodium is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.
Large-Scale Manufacturing and Export
Grade Compliance
Cloprostenol sodium is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.