Industries across sectors rely on sodium acetate for its versatility and use it in two primary forms: Sodium Acetate Anhydrous and Sodium Acetate Trihydrate.
Although both share the same chemical base, their physical properties and moisture content lead them to serve different purposes.
Professionals in food processing, pharmaceuticals, textiles, and construction must understand these differences to ensure precision and maintain product purity.
In this blog, we’ll explore the differences between sodium acetate anhydrous and trihydrate in terms of chemical structure, properties, uses, manufacturing, handling, and storage—helping you make an informed decision when choosing the right form for your needs.
What is Sodium Acetate?
Sodium acetate is the sodium salt of acetic acid, with the chemical formula CH₃COONa. It appears as a white crystalline solid and is highly soluble in water. This compound offers buffering ability, exhibits mild alkalinity, and plays a vital role in various chemical, industrial, and biological applications.
Sodium acetate is available in two main forms:
- Sodium Acetate Anhydrous (without water)
- Sodium Acetate Trihydrate (with three molecules of water)
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
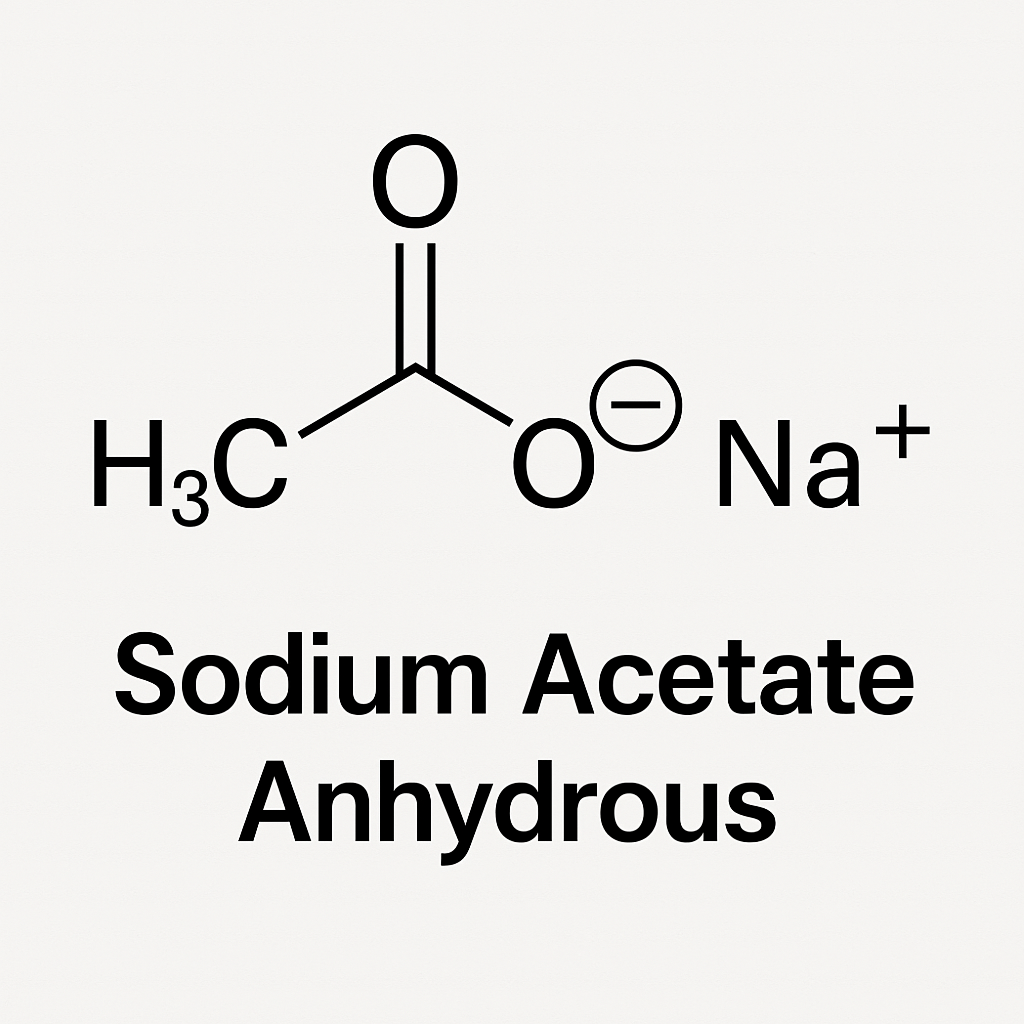
What is Sodium Acetate Anhydrous?
Sodium Acetate Anhydrous represents the water-free form of sodium acetate. Manufacturers prepare it by removing all water molecules during processing. This form suits applications that require the absence of moisture, including chemical synthesis, pharmaceutical formulations, and industrial reactions.
Key Features:
- Chemical Formula: CH₃COONa
- Molecular Weight: 82.03 g/mol
- Appearance: White crystalline powder
- Moisture Content: 0%
- Solubility: Highly soluble in water and ethanol
- Stability: More stable under dry conditions
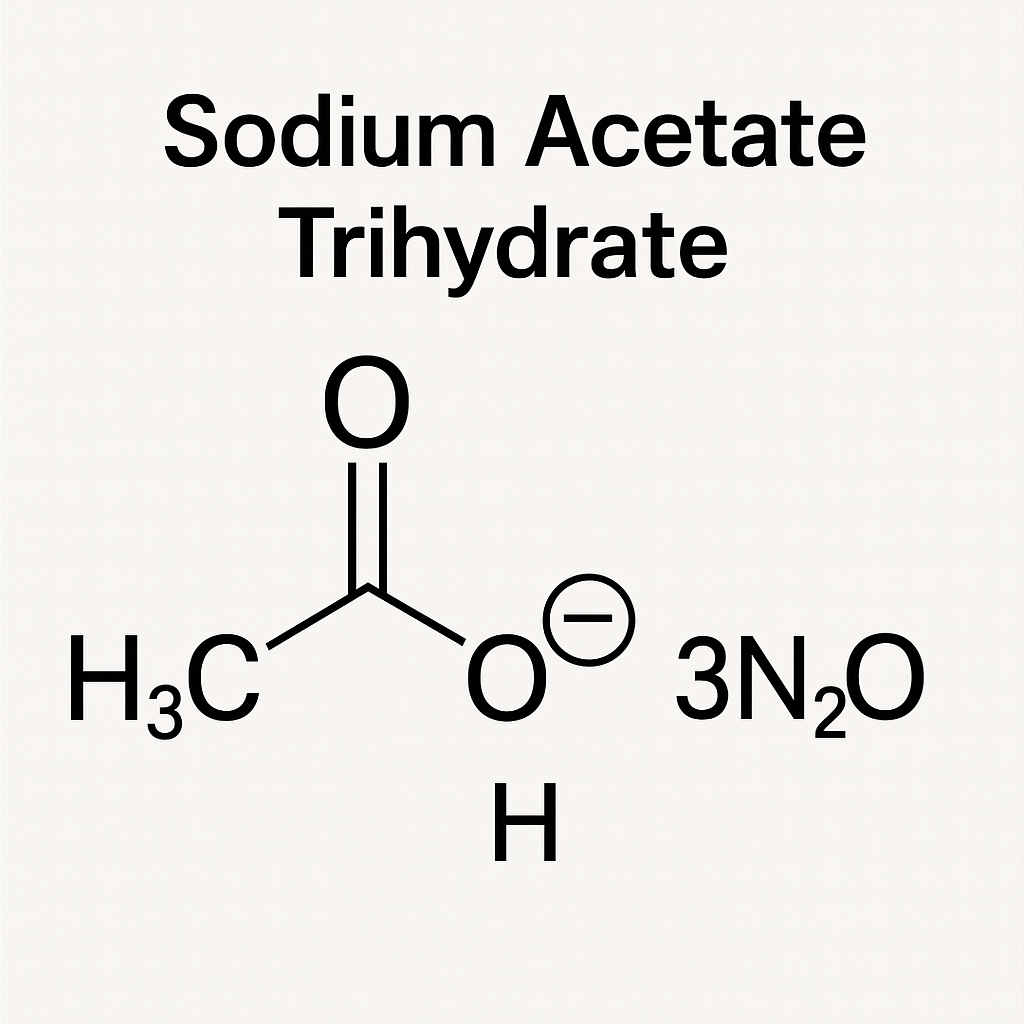
What is Sodium Acetate Trihydrate?
Sodium Acetate Trihydrate is the hydrated form of sodium acetate, meaning it contains three molecules of water (H₂O) for each sodium acetate unit. This water of crystallization gives it a different molecular weight and density, which affects its application and storage.
Key Features:
- Chemical Formula: CH₃COONa·3H₂O
- Molecular Weight: 136.08 g/mol
- Appearance: White crystalline solid or powder
- Moisture Content: ~37%
- Solubility: Highly soluble in water
- Stability: Stable in ambient conditions but can lose water upon heating
Chemical Comparison Table
| Feature | Sodium Acetate Anhydrous | Sodium Acetate Trihydrate |
|---|---|---|
| Chemical Formula | CH₃COONa | CH₃COONa·3H₂O |
| Molecular Weight | 82.03 g/mol | 136.08 g/mol |
| Appearance | White, dry crystalline powder | White crystalline solid |
| Water Content | 0% | ~37% |
| pH (1% solution) | ~8.9 | ~8.9 |
| Solubility | High | High |
| Density | Higher due to absence of water | Lower due to hydration |
| Shelf Life | Longer if stored dry | Sensitive to humidity |
How Are They Made?
Sodium Acetate Anhydrous Production
To produce sodium acetate anhydrous:
- React acetic acid with sodium carbonate or sodium hydroxide.
- The solution is filtered and evaporated to dryness.
- Crystals are then heated to remove all traces of moisture.
Sodium Acetate Trihydrate Production
To produce the trihydrate form:
- The same chemical reaction is performed between acetic acid and a sodium source.
- The resulting solution is crystallized at a controlled temperature, allowing it to bind with three water molecules.
- The crystals are dried but not heated beyond 58°C, to retain hydration.
Applications: Sodium Acetate Anhydrous vs. Trihydrate
Though both forms are chemically similar, they are preferred for different uses based on their water content and physical behavior.
Sodium Acetate Anhydrous Uses:
- Pharmaceutical industry – As a buffering agent in tablets and injections where water content must be minimized.
- Chemical synthesis – Used as a dehydrating agent and in reactions sensitive to moisture.
- Food processing – Used in dry seasoning mixes and additives (food-grade variant).
- Laboratory – For precise molar concentrations in biological research.
- Catalysis – As a reagent in organic reactions.
Sodium Acetate Trihydrate Uses:
- Textile industry – Acts as a buffer in dyeing and printing processes.
- Heat packs – Used in crystallization-based exothermic heat packs.
- Food industry – Preservative and flavoring agent (E262).
- Molecular biology – Precipitates DNA and RNA in lab protocols.
- Concrete sealing – Used in eco-friendly de-icing and moisture control solutions.
Physical & Chemical Behavior
Hydration Sensitivity
- Anhydrous form is highly sensitive to moisture. It readily absorbs water from the air and can turn into the trihydrate form if exposed for too long.
- Trihydrate is more stable under ambient conditions but can lose water when heated above 58°C, reverting to anhydrous.
Weight & Dosing
Due to the difference in molecular weight, the same amount of each compound contains different amounts of active sodium acetate. Accurate dosing is important in pharmaceuticals and lab research.
Crystallization & Handling
Trihydrate crystals are larger and may take longer to dissolve, especially at lower temperatures. Anhydrous dissolves faster and offers more precise measurements.
Cost Considerations
- Sodium Acetate Anhydrous is typically more expensive due to the additional processing involved in removing water.
- Sodium Acetate Trihydrate is more economical and easier to produce at scale, making it more common in general applications.
Storage & Shelf Life
Anhydrous:
- Must be stored in airtight containers to prevent moisture absorption.
- Has a longer shelf life if kept in dry conditions.
Trihydrate:
- Can tolerate normal humidity levels but must be protected from high heat.
- May degrade over time if exposed to open air, especially in hot environments.
Safety and Handling
Both forms are considered non-toxic and safe to handle when proper precautions are followed.
- Avoid inhaling dust from either form.
- Use gloves and eye protection during handling.
- Store away from strong acids, bases, and moisture-sensitive chemicals.
Environmental Impact
Sodium acetate is biodegradable and considered environmentally friendly. It is often used as an alternative to environmentally harmful de-icing salts like calcium chloride.
- Trihydrate is often used in green building and road maintenance.
- Anhydrous has limited direct environmental applications but is still eco-safe.
Which Form Should You Use?
| Application Area | Recommended Form |
|---|---|
| Pharmaceuticals | Anhydrous |
| Food Additives | Trihydrate (food-grade) |
| Heat Packs | Trihydrate |
| DNA/RNA Extraction | Trihydrate |
| Lab Reagents | Anhydrous |
| Dry Seasoning Blends | Anhydrous |
| Textile Dyeing | Trihydrate |
| De-icing & Concrete Sealing | Trihydrate |
Conclusion
While sodium acetate anhydrous and trihydrate share the same base chemical structure, their differences in water content, molecular weight, and handling properties make them suitable for very different purposes.
- If you’re working in an environment that requires precision, moisture control, or pharmaceutical-grade materials, sodium acetate anhydrous is your go-to choice.
- If your needs revolve around food, textiles, or heat generation, sodium acetate trihydrate is more cost-effective and easier to work with.
Choosing the right form of sodium acetate ensures better efficiency, stability, and accuracy across your processes.
Chemignition Laboratory is a trusted manufacturer, supplier, and exporter of high-quality Sodium Acetate Anhydrous and Sodium Acetate Trihydrate, serving various industries with reliable and consistent chemical solutions.
FAQs
What is the difference between Sodium Acetate Anhydrous and Trihydrate?
Sodium Acetate Anhydrous contains no water molecules, while Sodium Acetate Trihydrate contains three water molecules per formula unit.
Do you provide bulk and customized packaging options?
Yes. We offer bulk quantities, customized packaging, and private labeling to meet the specific needs of domestic and international clients.
Are your products certified?
Yes. All our products adhere to industry standards and come with COA (Certificate of Analysis), MSDS, and other required documentation upon request.
What is your export capability?
We export globally and ensure safe, compliant, and timely shipments with proper documentation and packaging, meeting all international standards.
Can I request a sample before placing a bulk order?
Yes. Chemignition Laboratory provides samples for quality testing upon request.
Do you offer technical support or usage guidance?
Absolutely. Our experts can guide you on product applications, handling instructions, and safety protocols tailored to your industry.