Glaucoma, one of the leading causes of irreversible blindness worldwide, affects more than 76 million people globally. Managing intraocular pressure (IOP) effectively is critical to slowing disease progression and protecting vision. Among the first-line drugs approved for IOP reduction is Bimatoprost, a synthetic prostamide and potent ophthalmic Active Pharmaceutical Ingredient (API).
In this comprehensive guide, we explore the role of Bimatoprost in glaucoma treatment, including its mechanism of action, efficacy, safety, and why it’s the preferred choice for formulators and ophthalmic pharmaceutical brands worldwide.
👁️🗨️ 1. Understanding Glaucoma and the Need for IOP Control
Glaucoma is a group of optic neuropathies characterized by the degeneration of the optic nerve and retinal ganglion cells. The primary risk factor? Elevated intraocular pressure (IOP).
Types of Glaucoma:
- Open-angle glaucoma (most common)
- Angle-closure glaucoma
- Normal-tension glaucoma
- Secondary glaucoma (trauma, steroid-induced)
IOP elevation results from impaired outflow of aqueous humor, the fluid that nourishes the eye and maintains pressure.
Uncontrolled IOP damages the optic nerve, leading to progressive vision loss.
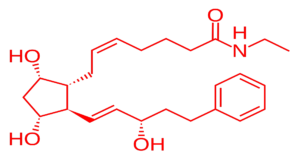
💊 2. Introduction to Bimatoprost
Bimatoprost is a prostaglandin F2α (PGF2α) analog, structurally classified as a prostamide. It is used as a first-line medication for treating:
- Primary open-angle glaucoma (POAG)
- Ocular hypertension (OHT)
Brand Examples:
- Lumigan® (Allergan) – Bimatoprost 0.01% / 0.03%
- Generic formulations – 0.03% ophthalmic solution
🔬 3. Mechanism of Action: How Bimatoprost Lowers IOP
Unlike beta-blockers or alpha-agonists, Bimatoprost increases aqueous humor outflow, not by reducing production but by enhancing drainage via two distinct pathways:
| Pathway | Effect |
|---|---|
| Uveoscleral outflow | Main site of action – enhanced flow through ciliary body |
| Trabecular meshwork | Secondary mechanism – improved fluid egress |
This dual mechanism results in a 25–33% reduction in IOP, even in patients unresponsive to other drugs.
📊 4. Clinical Efficacy of Bimatoprost
Numerous trials confirm Bimatoprost’s superior and sustained IOP-lowering effect compared to other prostaglandin analogues:
Study Highlights:
- In randomized trials, Bimatoprost reduced IOP by 7–9 mmHg over 24-hour periods.
- Compared with Latanoprost or Travoprost, Bimatoprost often delivered slightly better peak IOP reduction.
Duration of Action:
- Once-daily application provides 24-hour control, making it convenient and effective for long-term use.
🧬 5. Pharmacokinetics and Dosage
| Property | Details |
|---|---|
| Formulation | Ophthalmic solution, 0.03% |
| Application | 1 drop in affected eye(s) daily |
| Onset of action | Within 4 hours |
| Peak IOP reduction | At 8–12 hours post dose |
| Half-life (systemic) | Approx. 45 minutes |
Systemic exposure is minimal with topical administration, making Bimatoprost safe and suitable for chronic use.
🧪 6. Bimatoprost API Profile for Manufacturers
As a GMP-compliant pharmaceutical API, Bimatoprost must meet stringent quality, purity, and safety standards.
Key Specifications:
- CAS No.: 155206-00-1
- Molecular Formula: C25H37NO4
- Molecular Weight: 415.58 g/mol
- Appearance: White to off-white crystalline powder
- Solubility: Practically insoluble in water, but it is soluble in organic solvents such as ethanol and methanol
👉 At Chemignition Laboratory, we provide high-purity, ready Bimatoprost API for regulated and emerging markets.
⚠️ 7. Side Effects and Safety Profile
Bimatoprost is generally well tolerated. However, like all ophthalmic drugs, mild side effects can occur.
Common Adverse Effects:
- Ocular redness (hyperemia)
- Increased pigmentation of the iris
- Eyelash growth (often desirable)
- Darkening of eyelid skin
- Mild eye irritation or dryness
Rare Effects:
- Conjunctival edema
- Periorbital fat atrophy
- Headache
Precautions:
- Not recommended in patients with active uveitis or macular edema
- Avoid contact with soft contact lenses during application
🌍 8. Regulatory Status and Market Demand
Bimatoprost is approved by:
- US FDA
- European Medicines Agency (EMA)
- TGA (Australia)
- DCGI (India)
It is included in:
- United States Pharmacopeia (USP)
- European Pharmacopoeia (EP)
Global Market Insight:
- Estimated CAGR of 5.2% (2023–2028)
- Growing usage in both ophthalmic and aesthetic applications
- Strong demand in North America, EU, Middle East, South America
🏭 9. Chemignition Laboratory: Trusted Bimatoprost API Manufacturer
As a GMP-certified API manufacturer, supplier, and exporter, Chemignition Laboratory provides pharmaceutical-grade Bimatoprost API to global clients in:
- Ophthalmology
- Generic formulation
- Contract manufacturing
- Aesthetic product development
Why Partner With Us?
- GMP-certified production facility
- Customizable bulk packaging
- Stability data, CoA, MSDS, and DMF support available
- Global export capabilities (US, EU, Latin America, MENA)
✅ Conclusion
Bimatoprost remains one of the most effective and widely accepted first-line treatments for glaucoma and ocular hypertension. With its dual mechanism of IOP reduction, long-term safety profile, and patient compliance, it continues to drive innovation in ophthalmic drug development.
Whether you’re developing a prescription formulation or sourcing raw API for export, quality and compliance are essential.
📢 Contact Chemignition Laboratory for GMP-certified Bimatoprost API today.
Bulk orders
Regulatory documents
Worldwide shipping
Sample availability
👉 Contact Us to request a quote or technical data sheet.
FAQs
Can I stop using Bimatoprost if my IOP is normal?
No, stopping Bimatoprost can cause IOP to rise again, increasing glaucoma risk. Always follow your doctor’s advice.
Does Bimatoprost change eye color permanently?
Yes, it can cause brown pigmentation of the iris, which may be permanent.
Is Bimatoprost better than Latanoprost?
Bimatoprost often lowers IOP more effectively but has a slightly higher risk of redness and pigmentation changes.
Can I use Bimatoprost for eyelash growth and glaucoma at the same time?
Yes, but using both applications separately is recommended to avoid contamination.
What is glaucoma?
Glaucoma is a group of eye diseases that can lead to damage of the optic nerve.
What are prostaglandin analogues?
Prostaglandin (PG) analogues are a new class of ocular hypotensive drugs that have been developed for the treatment of open angle glaucoma.