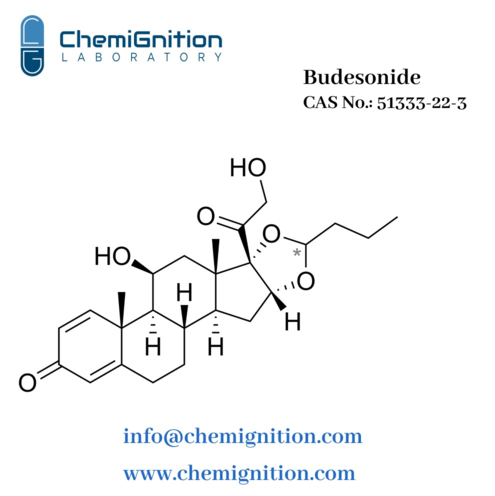
Key Properties of Budesonide
| Product Name: | Budesonide |
| Synonyms: | (RS)-11β,21-dihydroxy-16α,17-[(1RS)-butylidenebis(oxy)]-pregna-1,4-diene-3,20-dione |
| CAS No: | 51333-22-3 |
| Chemical Formula: | C₂₅H₃₄O₆ |
| Grade: | BP/EP |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 430.53 g/mol |
| Solubility: | Sparingly soluble in water, but soluble in ethanol and methanol. |
Manufacturing process of Budesonide
The manufacturing process of Budesonide API begins with a thorough inspection of equipment to ensure cleanliness, followed by verification of the availability of all utilities, raw materials, and packing materials required for the process.
The preparation stage, a suitable solvent such as acetone or isopropanol is charged into a glass-lined reactor, and starting materials like 16α, 17α-acetal of prednisolone are added under continuous stirring while maintaining the temperature at 20–25°C.
During the reaction stage, a reaction agent (e.g., acid catalyst or oxidizing agent) is gradually added, and the temperature is increased to 50–55°C to initiate the reaction. The reaction is maintained for 360–420 minutes with continuous stirring, and progress is monitored using in-process tests such as HPLC.
Once the reaction is complete, the mixture is cooled to 20–25°C, and an aqueous solution (e.g., sodium chloride or water) is added to quench the reaction. The organic and aqueous layers are then separated using a separation funnel. The organic layer is concentrated under reduced pressure to obtain a crude product, which is further purified using recrystallization with solvents such as ethanol or methanol. The purified solution is heated to dissolve the product completely, then cooled gradually to 0–5°C to promote crystallization. The crystals are stirred for 120–240 minutes to enhance formation, filtered using a vacuum filter or centrifuge, and washed with a chilled solvent to remove impurities.
The wet product is transferred to a vacuum dryer and dried at 40–50°C under vacuum for 480–600 minutes to achieve the desired moisture content. The dried product is milled to achieve uniform particle size and sieved through a specified mesh for consistency.
Finally, Pack Budesonide in a clear poly bag and twist the mouth of the bag. Secure it with a PVC strip. Place this bag inside another poly bag, twist its mouth, and secure it with a PVC strip. Insert these bags into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Budesonide in a tightly closed, light-resistant container at a temperature below 30°C.
Applications of Budesonide
Technical Specifications of Budesonide
| Sr.No. | Test | Specification |
|---|---|---|
| 1 | Description | A white or almost-white, crystalline powder. |
| 2 | Solubility | Freely soluble in dichloromethane; sparingly soluble in ethanol (95%). |
| 3 | Loss on Drying | Not more than 0.5% (at 105°C for 3 hours). |
| 4 | Identification: By IR Spectrophotometer | The Infrared spectrum of sample preparation should concord with the standard preparation of Budesonide CRS / WS. |
| 5 | Identification: By Thin-layer Chromatography | The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a) at 254 nm. |
| 6 | Related Substances (by HPLC) – Any Other Impurity | Not more than 0.5%. |
| 7 | Related Substances (by HPLC) – Total of All Impurities | Not more than 1.5%. |
| 8 | Assay (by HPLC) | Not less than 98.0% and not more than 102.0% (on dried basis). |
| 9 | Residual Solvents (by GC) | Methanol: Not more than 3000 ppm DIPE: Not more than 5000 ppm 1,4-Dioxane: Not more than 380 ppm Methylene Dichloride: Not more than 600 ppm |
Packing and Storage condition of Budesonide
Pack Budesonide in a clear poly bag and twist the mouth of the bag. Secure it with a PVC strip. Place this bag inside another poly bag, twist its mouth, and secure it with a PVC strip. Insert these bags into a triple-laminated aluminum bag and seal it properly. Finally, place the sealed aluminum bag in an HDPE container. Store Budesonide in a tightly closed, light-resistant container at a temperature below 30°C.
Why Choose Chemignition for
Budesonide Supply?
Quality Assurance
Grade Compliance
Budesonide is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.
Large-Scale Manufacturing and Export
Grade Compliance
Budesonide is manufactured in adherence to stringent global pharmaceutical standards.
In-House Quality Control
Each batch undergoes rigorous testing for purity, stability, and efficacy.
Regulatory Approvals
Certified for export and compliant with international regulatory requirements.