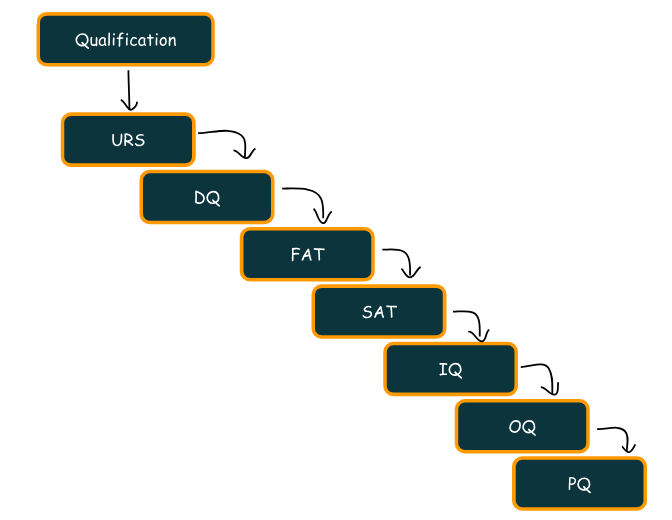
“Qualification in the Pharmaceutical Industry: Ensuring Safety and Compliance”
What is Qualification? Qualification establishes confidence that process equipment and ancillary systems consistently operate within established limits and tolerances. In addition, it provides documented evidence that equipment has been installed according to specifications (manufacturer’s recommendations) and will reliably maintain critical process parameters. In the pharmaceutical industry, qualification is vital. Specifically, it ensures that equipment, systems,…