Tafluprost is a prostaglandin analog used to manage elevated intraocular pressure in patients with open-angle glaucoma and ocular hypertension. Its efficacy in reducing intraocular pressure, coupled with its preservative-free formulation, makes it a preferred treatment option for individuals with sensitivity to preservatives. This guide explores the structure, properties, pharmacology, uses, and safety of Tafluprost.
1. Structure of Tafluprost
The molecular structure of Tafluprost underpins its ability to enhance aqueous humor outflow and effectively lower intraocular pressure.
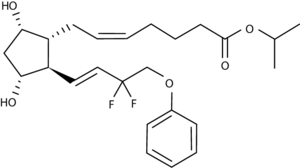
2D Structure
The 2D structure of Tafluprost features a prostaglandin F2α analog backbone with ester and fluorine substitutions that improve its stability and receptor-binding affinity.
3D Structure
The 3D conformation of Tafluprost reveals its ability to selectively bind to FP receptors in the ciliary muscle, increasing the outflow of aqueous humor through the uveoscleral pathway.

2. Names and Identifiers
Tafluprost is recognized under various names and identifiers in medical and pharmacological databases.
- IUPAC Name:
Isopropyl (Z)-7-{(1R,2R,3R,5S)-2-[[(1E)-3,3-difluoro-4-phenoxybut-1-en-1-yl]oxy]-3,5-dihydroxycyclopentyl}hept-5-enoate - Molecular Formula:
C25H34F2O5 - Molecular Weight:
452.6 g/mol - CAS Registry Number:
209860-87-7 - Synonyms:
Zioptan, Taflotan
Explore a leading manufacturer of APIs.
With over 10 years of expertise, we ensure GMP compliance and provide reliable, high-quality solutions.
3. Chemical and Physical Properties
The chemical and physical properties of Tafluprost contribute to its therapeutic efficacy and stability.
| Property | Details |
|---|---|
| Appearance | Clear, colorless to slightly yellow liquid |
| Melting Point | Not applicable (liquid form) |
| Solubility | Soluble in ethanol and methanol; sparingly soluble in water |
| LogP (Partition Coefficient) | ~3.9 |
| Chemical Class | Prostaglandin Analog |
4. Drug and Medication Information
Tafluprost is primarily used to lower intraocular pressure in patients with glaucoma or ocular hypertension.
Formulations
- Ophthalmic Solution:
- Available as 0.0015% eye drops, often in preservative-free single-dose vials.
Mechanism of Action
Tafluprost works by:
- Increasing Uveoscleral Outflow: Enhances the drainage of aqueous humor from the eye, reducing intraocular pressure.
- Targeting FP Receptors: Activates prostaglandin receptors in the ciliary muscle to facilitate fluid outflow.
Dosage and Administration
- Recommended Dose:
- Apply one drop in the affected eye(s) once daily in the evening.
Avoid overuse, as it may reduce effectiveness.
- Apply one drop in the affected eye(s) once daily in the evening.
5. Pharmacology and Biochemistry
Tafluprost’s pharmacology emphasizes its role in managing elevated intraocular pressure.
Pharmacokinetics
- Absorption:
Rapidly absorbed through the cornea following topical application. - Onset of Action:
Significant intraocular pressure reduction observed within 2-4 hours, with peak effects at 12 hours. - Half-Life:
Approximately 30 minutes in aqueous humor. - Excretion:
Primarily eliminated via urine as inactive metabolites.
Pharmacodynamics
Tafluprost mimics prostaglandin F2α activity, increasing aqueous humor outflow through the uveoscleral pathway. Its localized action minimizes systemic effects.
6. Uses and Side Effects
Primary Uses
- Open-Angle Glaucoma:
Lowers intraocular pressure to prevent optic nerve damage. - Ocular Hypertension:
Reduces elevated intraocular pressure to prevent vision loss.
Benefits
- Effective in reducing intraocular pressure with once-daily dosing.
- Preservative-free formulation suitable for sensitive individuals.
- Minimal systemic absorption ensures a favorable safety profile.
Side Effects
Common side effects include:
- Eye redness or irritation.
- Increased pigmentation of the iris and eyelids.
- Eyelash growth or darkening.
Rare but serious side effects:
- Macular edema.
- Severe eye pain or vision changes.
Consult your doctor if side effects persist or worsen.
7. Safety and Hazards
Safety Profile
Tafluprost is safe for most patients when used as directed. However:
- Contraindications: Not suitable for patients with hypersensitivity to Tafluprost or its components.
- Pregnancy and Breastfeeding: Use with caution and only under medical supervision.
Chemical Safety

Handling Precautions
- Store in the refrigerator before opening. Once opened, store at room temperature and use within 30 days.
- Avoid touching the dropper tip to prevent contamination.
Environmental Impact
Tafluprost is biodegradable and poses minimal environmental risks when disposed of according to guidelines.
FAQs
IUPAC Name of Tafluprost?
Isopropyl (Z)-7-{(1R,2R,3R,5S)-2-[[(1E)-3,3-difluoro-4-phenoxybut-1-en-1-yl]oxy]-3,5-dihydroxycyclopentyl}hept-5-enoate.
Molecular Formula of Tafluprost?
C25H34F2O5.
Molecular Weight of Tafluprost?
452.53 g/mol
CAS Number of Tafluprost?
209860-87-7
What is Tafluprost used for?
Tafluprost is used to treat:
- Open-Angle Glaucoma: Reduces intraocular pressure to prevent optic nerve damage.
- Ocular Hypertension: Lowers elevated eye pressure to reduce the risk of vision loss.
How does Tafluprost work?
Tafluprost works by increasing the outflow of aqueous humor through the uveoscleral pathway. It activates prostaglandin F2α receptors in the eye’s ciliary muscle to enhance fluid drainage and lower intraocular pressure.
How is Tafluprost administered?
Tafluprost is applied as eye drops.