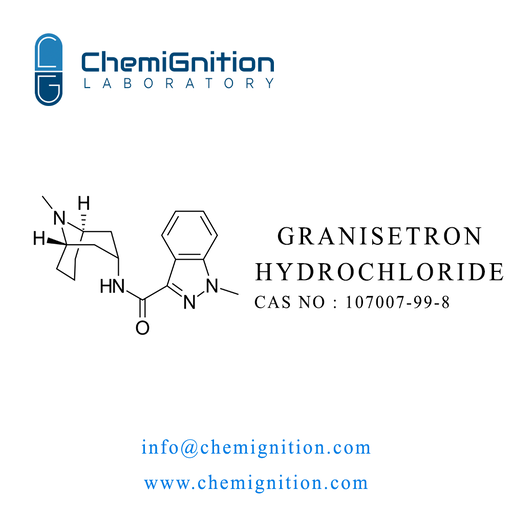
| Product Name: | Granisetron Hydrochloride |
| Synonyms: | 1-methyl-N-(9-methyl-9-azabicyclo[3.3.1]nonan-3-yl)indazole-3-carboxamide hydrochloride |
| CAS No: | 107007-99-8 |
| Chemical Formula: | C₁₈H₂₅ClN₄O |
| Grade: | USP |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 348.83 g/mol |
| Solubility: | Soluble in water and slightly soluble in ethanol. |
Description
Chemignition is a leading manufacturer and exporter of Granisetron Hydrochloride Active Pharmaceutical Ingredients (API) worldwide. The company adheres to strict Quality Management Systems (QMS) to ensure compliance with Good Manufacturing Practices (cGMPs). This commitment to quality and regulatory standards guarantees that Chemignition produces Granisetron Hydrochloride of the highest quality and efficacy.
Healthcare providers use Granisetron Hydrochloride as an antiemetic to prevent vomiting and nausea, commonly administering it after chemotherapy and post-anesthesia to mitigate these symptoms.
Chemignition employs various processes during the manufacturing of Active Pharmaceutical Ingredients (API) and specialty chemicals, including dissolution, charcoalization, filtration, leaching, extraction, crystallization, and purification.
Manufacturers commonly use Granisetron Hydrochloride API in pharmaceutical products such as injections, tablets, and capsules.
The U.S. Food and Drug Administration (FDA) approved Granisetron Hydrochloride in 1993 for preventing nausea and vomiting associated with chemotherapy, establishing its safety and efficacy for patients.
Storage Conditions: Pack Granisetron Hydrochloride in a double polybag, keep it in an HDPE or fiber drum, and store it at a temperature between 20°C and 25°C.