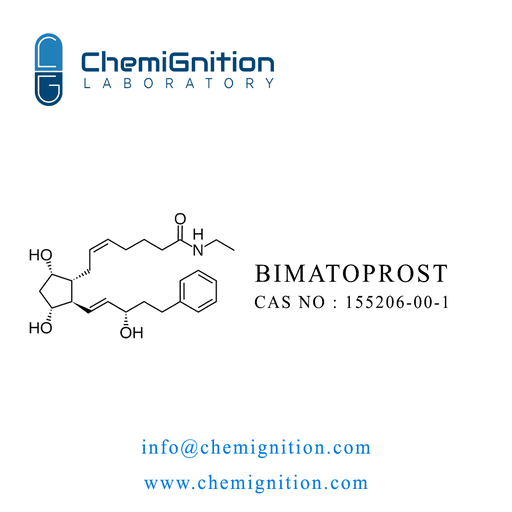
| Product Name: | Bimatoprost |
| Synonyms: | (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide |
| CAS No: | 155206-00-1 |
| Chemical Formula: | C₂₅H₃₇NO₄ |
| Grade: | USP/IP/IH |
| Appearance: | White to off-white crystalline powder |
| Molar Mass: | 415.58 g/mol |
| Solubility: | Practically insoluble in water, but it is soluble in organic solvents such as ethanol and methanol |
Description
Chemignition leads the global market as a manufacturer and exporter of Bimatoprost Active Pharmaceutical Ingredients (API). They strictly follow Quality Management Systems (QMS) to comply with Good Manufacturing Practices (cGMPs). This commitment to quality and regulatory standards ensures that Chemignition produces Bimatoprost with the highest quality and efficacy.
Bimatoprost treats eye diseases, specifically glaucoma and ocular hypertension. It repairs damage to the optic nerve (located at the back of the eye) and reduces abnormalities in the eye’s drainage system, preventing excessive pressure.
Chemignition conducts processes such as dissolution, charcoalization, filtration, leaching, extraction, crystallization, and purification during the manufacturing of Active Pharmaceutical Ingredients (API) and specialty chemicals.
Manufacturers commonly use Bimatoprost API in pharmaceutical products like ophthalmic solutions (eye drops). The U.S. Food and Drug Administration (FDA) approved Bimatoprost in 2001 for treating glaucoma and ocular hypertension, establishing its safety and efficacy.
Storage Conditions: Pack Bimatoprost in tightly closed vials and maintain it in a sealed container, storing it at temperatures between -20°C and -10°C